Figure 2.
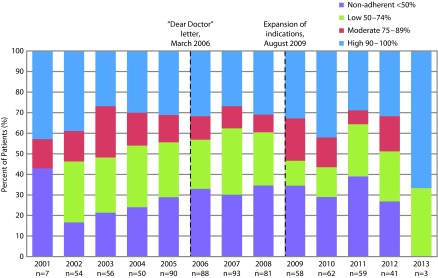
Temporal changes in adherence by calendar year* of the index date and by adherence periods of LFTs as defined by FDA inquiries and action† within the Optum Research Database from November 20, 2001 to March 31, 2013.
*Based on 40-day intervals; adherence per patient was categorized into the year of the patient’s date of initiation. †Regulatory events of interest during the study period include a Dear Doctor letter issued in March 2006 and expansion of indications to include milder forms of PAH in August 2009.
FDA, Food and Drug Administration; LFT, liver function test; REMS, risk evaluation mitigation strategies.
