Abstract
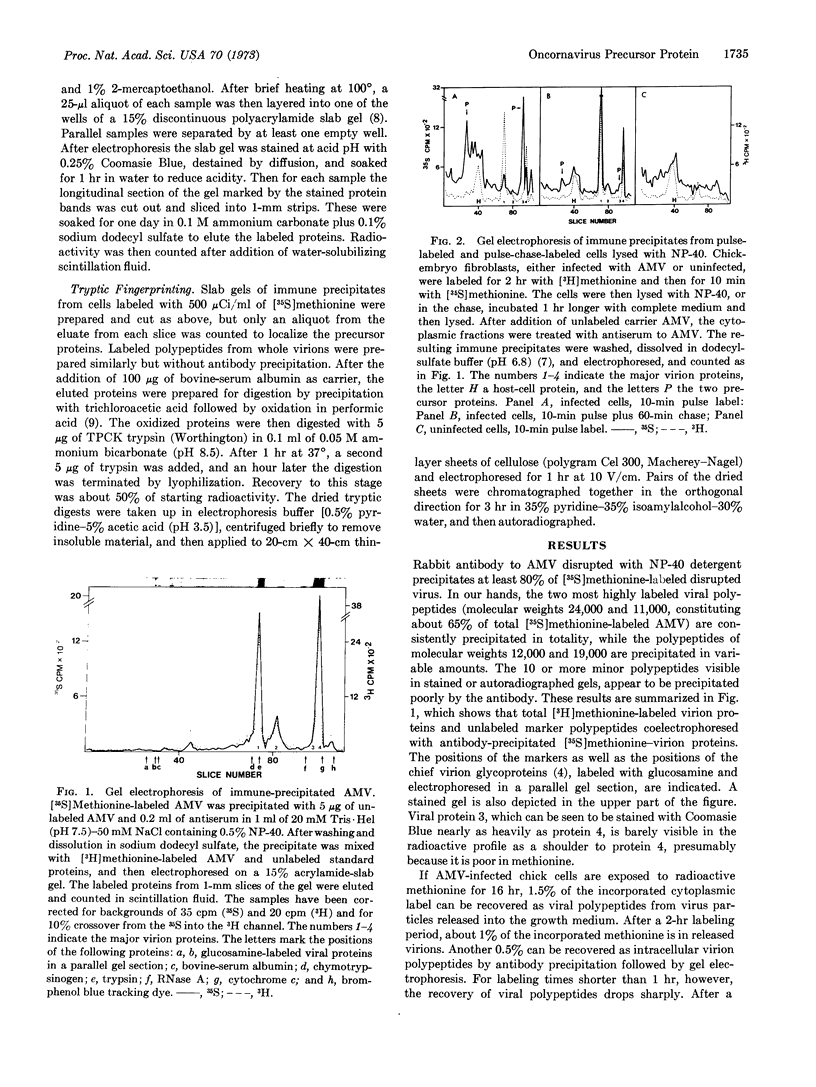
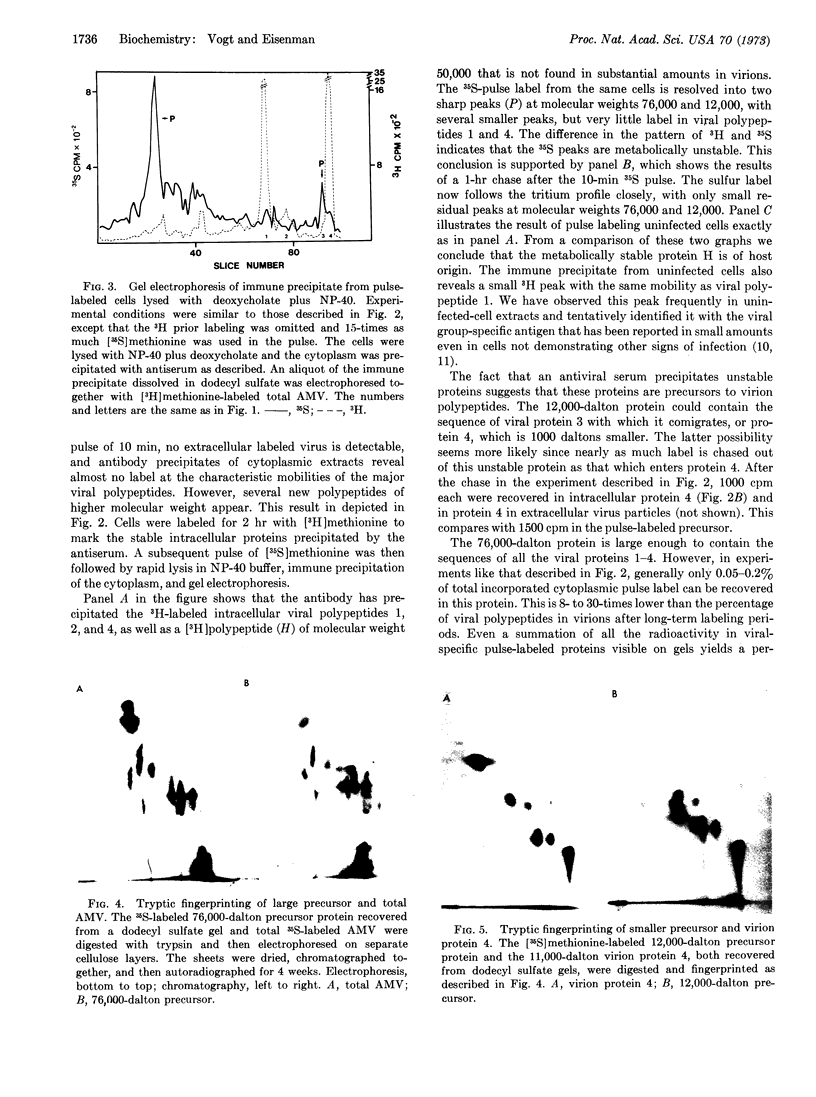
Antibody to partially disrupted avian myeloblastosis virus was used to selectively precipitate newly synthesized intracellular viral polypeptides from extracts of infected chicken cells. When analyzed by sodium dodecyl sulfate-gel electrophoresis, immune precipitates from extracts of cells pulse-labeled for 10 min with [35S]methionine contain none of the major virion polypeptides. Instead they show prominent viral specific polypeptides of molecular weight 76,000 and 12,000, as well as minor quantities of other labeled polypeptides. From pulse-chase kinetics and two-dimensional tryptic finger-prints it appears that the large polypeptide is a precursor of at least the two major virion proteins of molecular weights 24,000 and 11,000, while the smaller is a precursor of the 11,000-dalton virion protein.
Keywords: proteolytic cleavage, pulse chase, antibody, tryptic fingerprint
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Jacobson M. F., Asso J., Huang A. S. The formation of poliovirus proteins. Cold Spring Harb Symp Quant Biol. 1969;34:741–746. doi: 10.1101/sqb.1969.034.01.083. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L.J., Salden M. H.L., Bloemendal H., Konings R. N.H. Translation of oncogenic viral RNA and eukaryotic messenger RNA in the E. coli cell-free system. FEBS Lett. 1972 Dec 15;28(3):348–352. doi: 10.1016/0014-5793(72)80747-6. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ARMSTRONG D., OKUYAN M., SARMA P. S., TURNER H. C. SPECIFIC COMPLEMENT-FIXING VIRAL ANTIGENS IN HAMSTER AND GUINEA PIG TUMORS INDUCED BY THE SCHMIDT-RUPPIN STRAIN OF AVIAN SARCOMA. Proc Natl Acad Sci U S A. 1964 May;51:742–750. doi: 10.1073/pnas.51.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Vecchio G., Attardi D., Green M. Immunological studies on viral polypeptide synthesis in cells replicating murine sarcoma-leukemia virus. J Virol. 1972 Sep;10(3):447–455. doi: 10.1128/jvi.10.3.447-455.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert W., Konings R. N., Bauer H., Hofschneider P. H. Translation of avian myeloblastosis virus RNA in a cell-free lysate of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):888–891. doi: 10.1073/pnas.69.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]