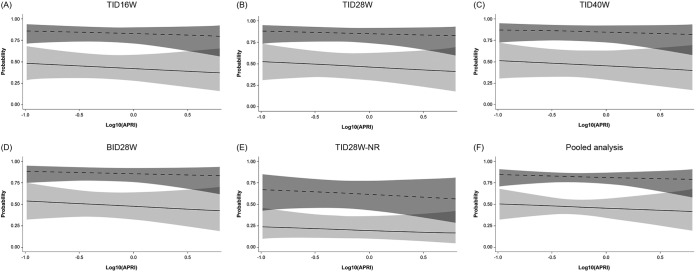
FIG 3.
Probability of SVR12, with 95% confidence limits, in patients with HCV GT-1a (solid lines) or GT-1b (dashed lines) infection according to APRI score. For the pooled analysis, the plot is based on a multiple logistic regression model of SVR12 versus log10(APRI) and genotype, with data restricted to ribavirin-containing groups. Odds ratios (ORs), 95% confidence intervals (CIs), and P values are outlined below. APRI, aspartate aminotransferase/platelet ratio index; BID, twice daily; GT, genotype; NR, no ribavirin; SVR12, sustained virologic response 12 weeks after completion of treatment; TID, three times daily. For the multiple regression model detailed in panels A to E, OR (95% CI) and P value for increasing APRI by 1 log were 0.77 (0.31, 1.95) and 0.5852, respectively. The ORs (95% CIs) and P value for GT-1a versus GT-1b in this model were 0.15 (0.09, 0.26) and < 0.0001 and for treatment TID16W versus TID28W were 0.85 (0.39, 1.86) and 0.6916, respectively; those for TID40W versus TID28W were 0.97 (0.42, 2.23) and 0.9469, respectively; those for BID28W versus TID28W were 1.07 (0.48, 2.35) and 0.8750, respectively; those for TID28W-NR versus TID28W were 0.28 (0.11, 0.71) and 0.0071, respectively. For the multiple regression models detailed in panel F, pooled data (excluding the no-RBV arm) are used. OR (95% CI) and P value for increasing APRI by 1 log were 0.81 (0.30, 2.20) and 0.6805, respectively. The OR (95% CI) and P value for GT-1a versus GT-1b in this model were 0.16 (0.09, 0.29) and <0.0001, respectively.