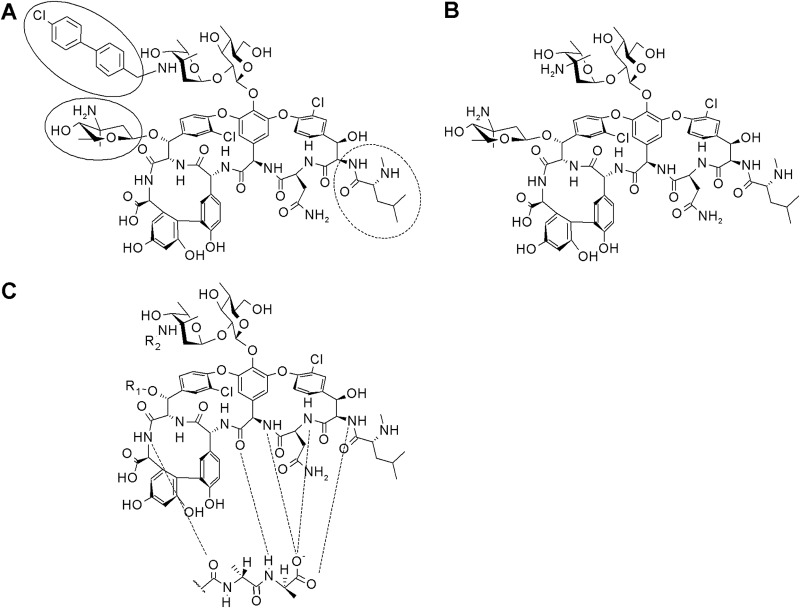
FIG 1.
Chemical structure of oritavancin (A), chloroeremomycin (B), and the glycopeptide backbone (C). The hydrophobic 4′-chlorobiphenyl methyl moiety and the 4-epi-vancosamine substituent are highlighted. The leucyl residue missing in des-N-methylleucyl-oritavancin is marked by a dashed circle. Hydrogen bonds are marked by dashed lines.