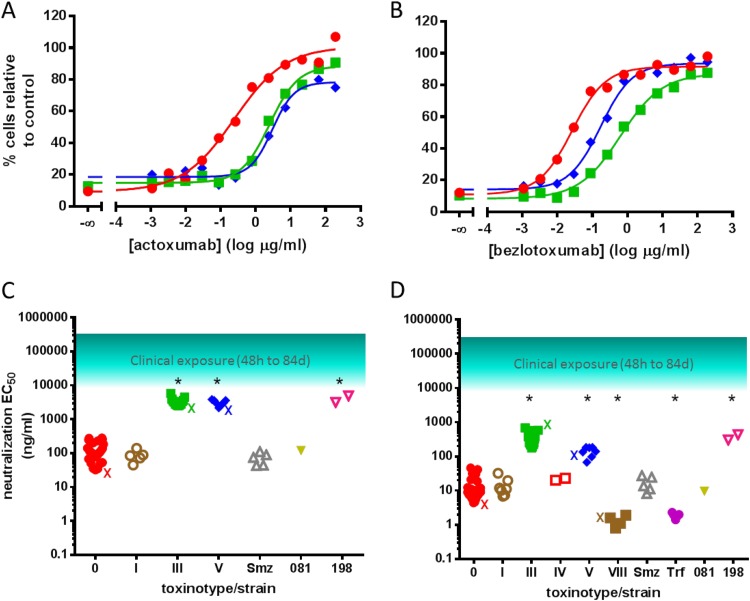
FIG 4.
Neutralization of TcdA and TcdB from culture supernatants of various clinical isolates by actoxumab and bezlotoxumab. (A and B) Actoxumab-mediated (A) and bezlotoxumab-mediated (B) neutralization of TcdA (T84 cells) and TcdB (Vero cells), respectively, from culture supernatants of strains of ribotypes 087 (strain VPI 10463) (red circles), 027 (strain 89 from tgcBIOMICS) (see Table S1 in the supplemental material) (green squares), and 078 (strain 73 from tgcBIOMICS) (see Table S1 in the supplemental material) (blue diamonds). (C and D) Actoxumab (C) and bezlotoxumab (D) EC50s against toxins in culture supernatants of all clinical isolates tested (Table 3; also see Table S1 in the supplemental material), grouped by toxinotype. The approximate range of serum antibody concentrations measured in patients participating in phase II clinical studies (7) is also shown. X, EC50 of purified toxins corresponding to each toxinotype (Table 1). *, P < 0.0001, compared to toxinotype 0.