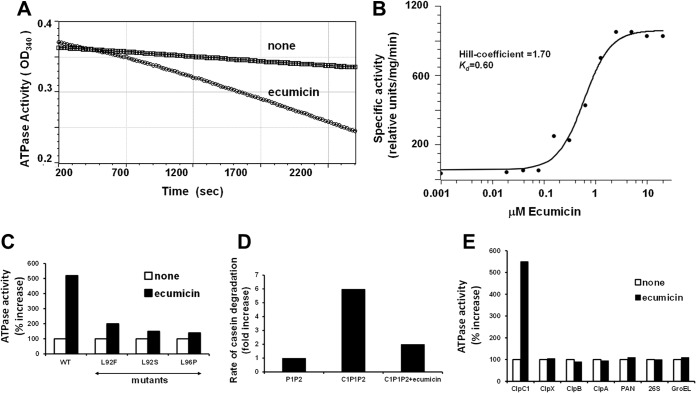
FIG 7.
Ecumicin selectively activates ATPase activity of wild-type ClpC1, but not protein degradation by the ClpC1P1P2 complex. (A) Ecumicin enhances the ATPase activity of wild-type ClpC1 derived from M. tuberculosis. Similar results were obtained when the ATPase activity was measured with the malachite green method. (B) Hill coefficient for activation of ClpC1 by ecumicin. The Kd and Hill coefficient for ecumicin activation of wild-type ClpC1 ATPase were determined using curve fitting with the classic Hill kinetic through a scaled Levenberg-Marquardt algorithm (tolerance 0.0001). (C) Ecumicin stimulates wild-type ClpC1 much more strongly than mutated ClpC1 from ecumicin-resistant strains. The ATPase activities of wild-type and mutant forms of ClpC1 (cloned and expressed in E. coli) are shown. The ATPase activity of each version of ClpC1 in the absence of ecumicin was taken as 100%. (D) ClpC1 (cloned and expressed in M. smegmatis) does not activate degradation of casein by ClpP1P2 in the presence of ecumicin. Each result is the average of three independent experiments. (E) Ecumicin activates M. tuberculosis ClpC1 but not several homologous AAA ATPases. The activities of purified AAA ATPases from several bacteria (M. tuberculosis ClpX; E. coli ClpA and ClpB), archaea (PAN), and mouse (26S proteasome) and the activity of the E. coli molecular chaperone GroEL were measured in the presence and absence of ecumicin (10 μM). The activity of each ATPase in the absence of ecumicin was taken as 100%. The deviations between the measurements were <5% for panels A, B, and C and <10% for panel D.