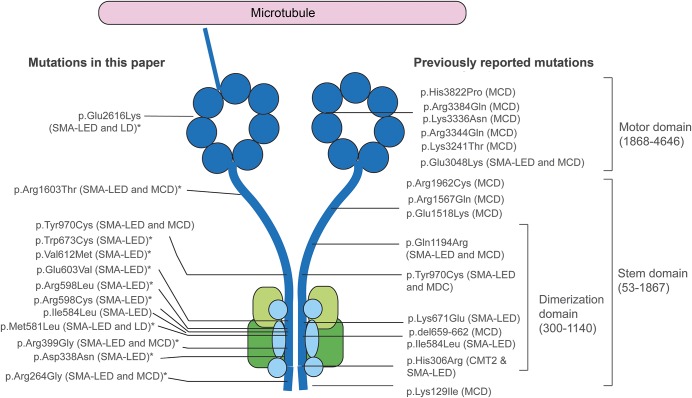
Figure 1. Position of mutations in DYNC1H1 relative to the structure of the dynein complex.
DYNC1H1 is a large gene encoding the heavy chain 1 of the cytoplasmic dynein protein complex, a ubiquitously expressed multisubunit molecular motor involved in retrograde axonal transport, cell migration, nucleokinesis, Golgi localization, and autophagy. The dynein complex consists of 2 heavy chains (dark blue), 2 intermediate chains (dark green), 4 light intermediate chains (light green), and a number of light chains (light blue). The tail domain, located in the N-terminus, is required for heavy chain dimerization. The dynein heavy chain motor domain (C-terminus) possesses adenosine triphosphate hydrolase activity and is required for movement along microtubules. This figure shows the position of all mutations described in this report and in the published literature. The mutations identified in this study to cause both SMA-LED and MCD can be seen to span the entire length of the protein. The cluster of mutations in the dimerization domain may be explained by the selective screening for mutations in this domain. *Novel mutation. CMT2 = Charcot-Marie-Tooth disease type 2; LD = learning disability with cognitive/behavioral impairment; MCD = malformation of cortical development; SMA-LED = spinal muscular atrophy with lower extremity predominance.