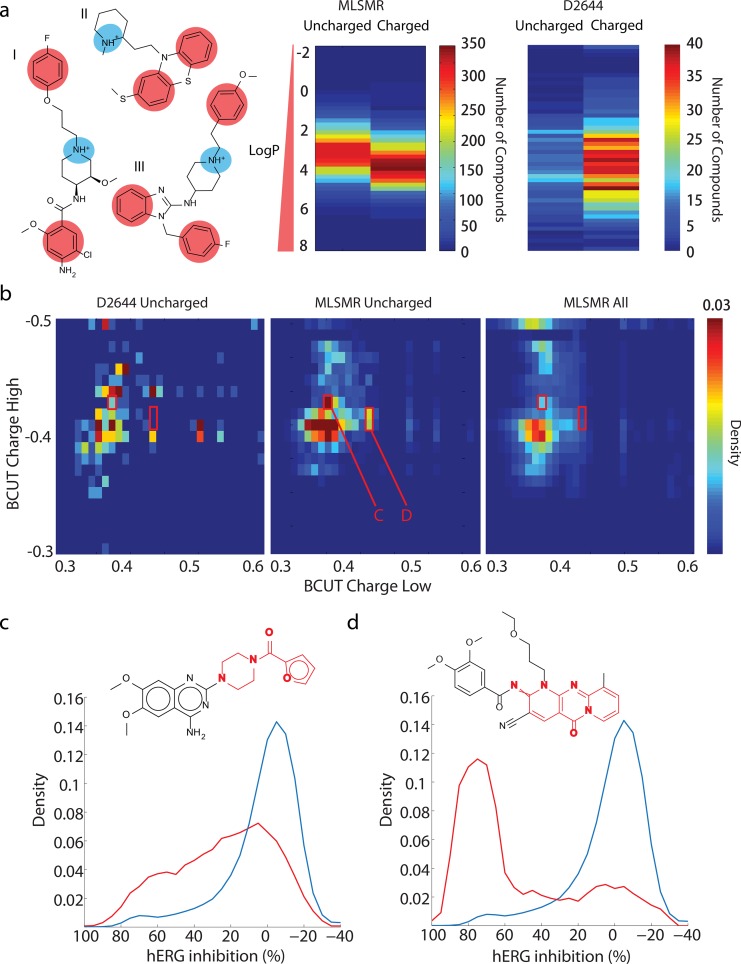
Fig 5. Novel structural determinants of hERG inhibitions.
(a) (Left) Classical charged hERG pharmacophore consisting of positively charged basic nitrogen (blue) and hydrophobic groups (red), demonstrated by cisapride (I), thioridazine (II) and astemizole (III). (Right) Distribution and density of LogP values for neutral and charged hERG blockers in D2644 collection (right) and MLSMR (left). (b) Density of chemical space mapped using largest and smallest BCUTTM charge descriptors for uncharged hERG blockers in D2644 (Left) and MLSMR (Center), and MLSMR library (Right). Red outlines denote enriched regions for neutral hERG blocker patterns in (c), (d). (c) Distribution of hERG inhibition for compounds containing prazosin fragment (red) compared to MLSMR library (blue). (d) As (c), for compounds containing illustrated triazatricyclo scaffold.