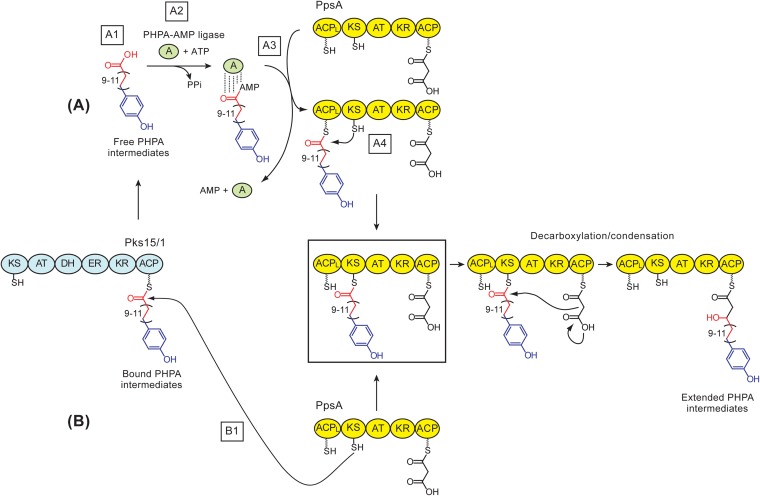
FIG 3.
Two possible mechanisms for transfer of p-hydroxyphenylalkanoate (PHPA) intermediates to PpsA in M. marinum. (A) PHPA-AMP ligase-dependent model. The model includes the release of PHPAs thioesterified to Pks15/1's ACP domain (A1), activation of free PHPAs by a PHPA-AMP ligase (A2), PHPA-AMP ligase-dependent loading of PHPAs onto PpsA's ACPL domain (A3), and capture of the ACPL domain-bound PHPAs by PpsA's KS domain to yield the fully loaded PpsA (boxed) ready for KS domain-dependent decarboxylation/condensation. (B) Direct KS domain capture model. In this model, the fully loaded PpsA (boxed) is generated directly by the capture of Pks15/1-bound PHPAs by PpsA's KS domain (B1), thus bypassing the need for steps A1 through A4. Decarboxylation/condensation leads to extension of PHPAs by a 2-carbon unit via the first noniterative extension cycle in the formation of phenolphthiocerols. In the scheme, the depicted carbon chain variability in the PHPA-Pks15/1 thioester intermediate is that expected during synthesis of M. marinum PGLs. Adenylated PHPAs are shown bound to the PHPA-AMP ligase via noncovalent linkages (by analogy to other acyl adenylating enzymes). PpsA's C-terminal ACP domain is shown loaded (AT domain dependent) with the malonyl-CoA-derived extender unit. Thiol groups of the phosphopantetheinyl group in the ACP domains and the catalytic Cys in the KS domain are depicted. The hydroxyl group in the extended PHPAs generated from the keto group by action of the KR domain of PpsA is shown. Sections of the PHPA intermediates are color coded based on origin: blue, derived from a p-hydroxybenzoic acid starter unit; red, derived from malonyl extender units via iterative extension cycles; black, section derived from a malonyl extender unit via a noniterative extension cycle. Domain abbreviations: A, adenylation; ACP, acyl carrier protein; ACPL, loading acyl carrier protein; AT, acyltransferase; DH dehydratase; ER, enoylreductase; KR, ketoreductase; KS, ketosynthase.