Figure 1.
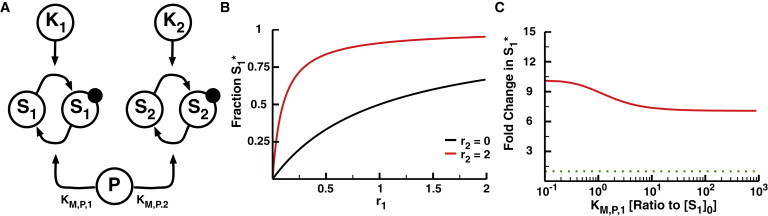
The 2-Kinase/1-Phosphatase loop. (A) Two independent kinases (K1 and K2) phosphorylate their respective substrates (S1 and S2). The solid circles indicate the phosphorylation of the substrates. A single shared phosphatase, P, dephosphorylates both substrates. (B) The fraction of phosphorylated S1 (denoted as S1∗) as a function of response parameter r1 when r2 = 0 (black) and r2 = 2 (red). r1 and r2 represent the ratio of the maximum velocity of the respective kinase to the maximum velocity of the phosphatase and are the dominant response parameters for the system. The initial concentrations of both S1 and S2 are set at 10 μM, KM,P,1 at 1 mM, and KM,P,2 at 1 μM. As such, S1 does not saturate either the kinase K1 or phosphatase, whereas S2 saturates K2 and P. Note the increase in phosphorylated S1 in response to activation of the second loop, as described previously for this motif (2). (C) The fold change in S1∗ as a function of KM,P,1. The fold change in S1∗ is calculated as the fraction S1∗ at r2 = 2 divided by the fraction S1∗ at r2 = 0. The dotted green line represents no change in S1∗. To see this figure in color, go online.
