Figure 2.
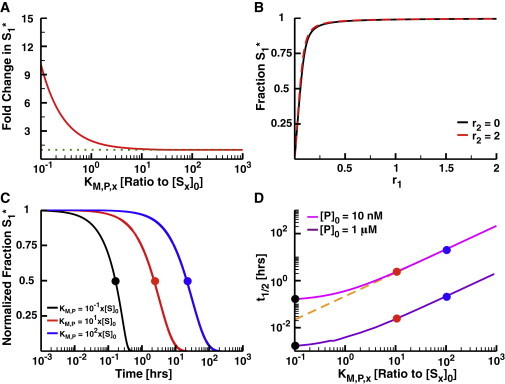
Removing coupling with unsaturatable phosphatases. (A) The fold increase in S1 phosphorylation as a function of the KM,P of the shared phosphatase for both substrates in a 2-Kinase/1-Phosphatase loop. (B) The fraction S1∗ as a function of r1 at r2 = 0 (black) and r2 = 2 (red) when KM,P = 10x[S]0. Note that there is very little difference between these curves. (C) The normalized fraction of phosphorylated substrate S1∗ as a function of time after the removal of input signal. In these simulations, the concentration of S2 and K2 are 0. The systems were allowed to run to steady state at high K1 activity (r1 = 2); at t = 0, the activity of the kinase was set to 0 (i.e., r1 = 0). The y axes were normalized by y1 = (y – miny) / (maxy – miny), where miny is the fraction S1∗ at r1 = 0 and maxy is the fraction S1∗ at r1 = 2 at steady state. (D) The half-life of S1 phosphorylation as a function of KM,P with two total concentrations of P (10 nM, green, and 1 μM, purple). Note that the black, red, and blue dots are shown to illustrate the relationship between (D) and (C). The dashed orange line shows the linear approximation of t1/2 for highly unsaturated phosphatases (t1/2 = KM,P/kcat,P[P]0). To see this figure in color, go online.
