Figure 5.
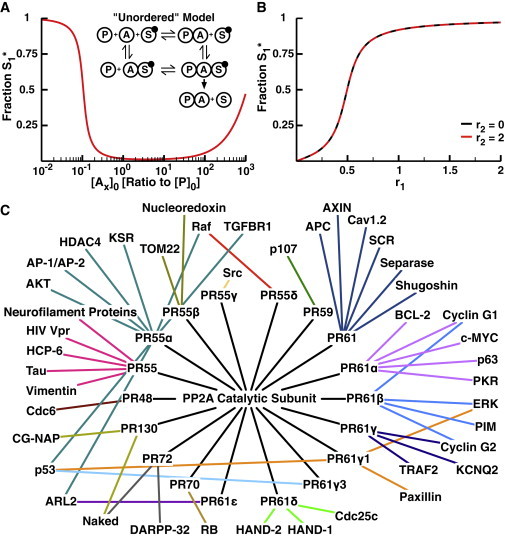
Unordered model of phosphatase regulatory adaptor subunits. (A) The fraction S1∗ as a function of initial concentration of each adaptor subunit with low levels of K1 activity (r1 = 0.05) and high levels of K2 activity (r2 = 2). Inset: diagram of the unordered adaptor model. In this case, the adaptor subunit can bind to the substrate without previously binding to the phosphatase (lower path). (B) The fraction S1∗ as a function of r1 at r2 = 0 (black) and r2 = 2 (red) with [Ax]0 = 100 nM. Note that this model provides insulation between the substrates. (C) Network diagram of the interactions of the PP2A catalytic subunit with each of 18 identified regulatory subunits (20). The PP2A holoenzyme formed by binding a regulatory subunit then interacts with a subset of the proteins in the network. To see this figure in color, go online.
