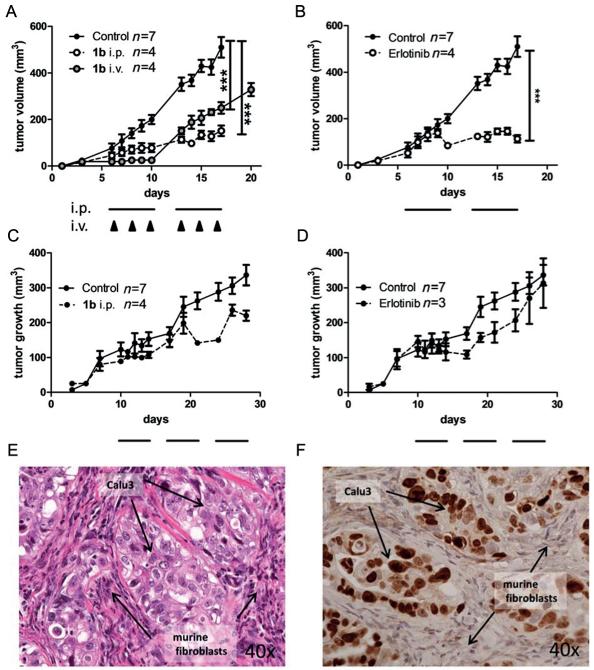
Figure 8.
In vivo anticancer activity of 1b and erlotinib in xenografts with human cancer cells. A431 (A, B) and Calu3 (C, D) were injected subcutaneously into the right flank of CB-17/SCID mice. When tumors were palpable, 1b was given as indicated. The applied dose was 5 mg kg−1 for i.v. and 25 mg kg−1 for i.p. application, respectively. Erlotinib was given at 25 mg kg−1 orally. For i.p. and oral therapy, cycles of five consecutive days are indicated by black bars, days of i.v. treatment are indicated by black arrows. Tumor volumes were calculated as described in the experimental section (Supporting Information). Data are means +/− SEM. Statistical significance tests: two-way ANOVA (*** p<0.001). On the last day of treatment the tumors were collected and histologically processed. Tissue morphology of untreated Calu3 xenografts was analyzed by E) H&E-stain and F) immunostain for human Ki67.