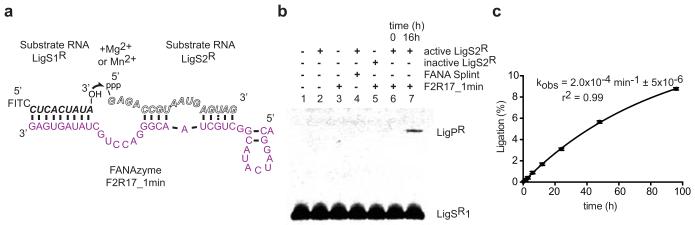
Figure 3. An RNA ligase XNAzyme (FANA).
a, Putative secondary structure of truncated chemically synthesized FANAzyme (F2R17_1min, purple) that ligates RNA substrate LigS1R to LigS2R, activated with 5′ triphosphate (ppp), in a trimolecular reaction in trans. b, Urea-PAGE gel showing no significant product (LigPR) observed with: substrate LigS1R alone (lane 1), no XNAzyme (lane 2), no LigS2R (lane 3), complementary FANA splint (lane 4), or LigS2R lacking 5′ppp (lane 5); product formation is dependent on LigS1R, activated LigS2R and XNAzyme (lanes 6 and 7). No product was detectable with combinations of RNA, DNA or FANA versions of LigS1 and (5′ppp)LigS2, except DNA LigS1 and RNA LigS2, which showed ~1.5% ligation after 20 h (Extended Data Fig. 7g). c, Pre-steady state trimolecular reaction rate (kobs) (n=3, error bars=sd, 25°C).