Abstract
Morphological transformation of normal rat kidney cells by a murine sarcoma virus was found to be cold-sensitive. Cells transformed by the virus expressed their transformed phenotype at the permissive temperature (39°) but not at the nonpermissive temperature (33°), as judged by the criteria of morphological changes and colony-forming ability on monolayers of normal rat kidney cells. Cold-sensitive expression of transformation was specific for focus-derived normal rat kidney cells transformed by the virus, readily reversible, and not lost during serial propagation of the cells. The genome of the murine sarcoma virus can be rescued by superinfection with Moloney leukemia virus at the permissive or nonpermissive temperature, and the rescued virus exhibited the same cold-sensitive properties as the original transforming virus. These results suggest that maintenance of the transformed state is continuously dependent on a cold-sensitive viral function.
Keywords: normal rat kidney cells, Moloney leukemia virus
Full text
PDF

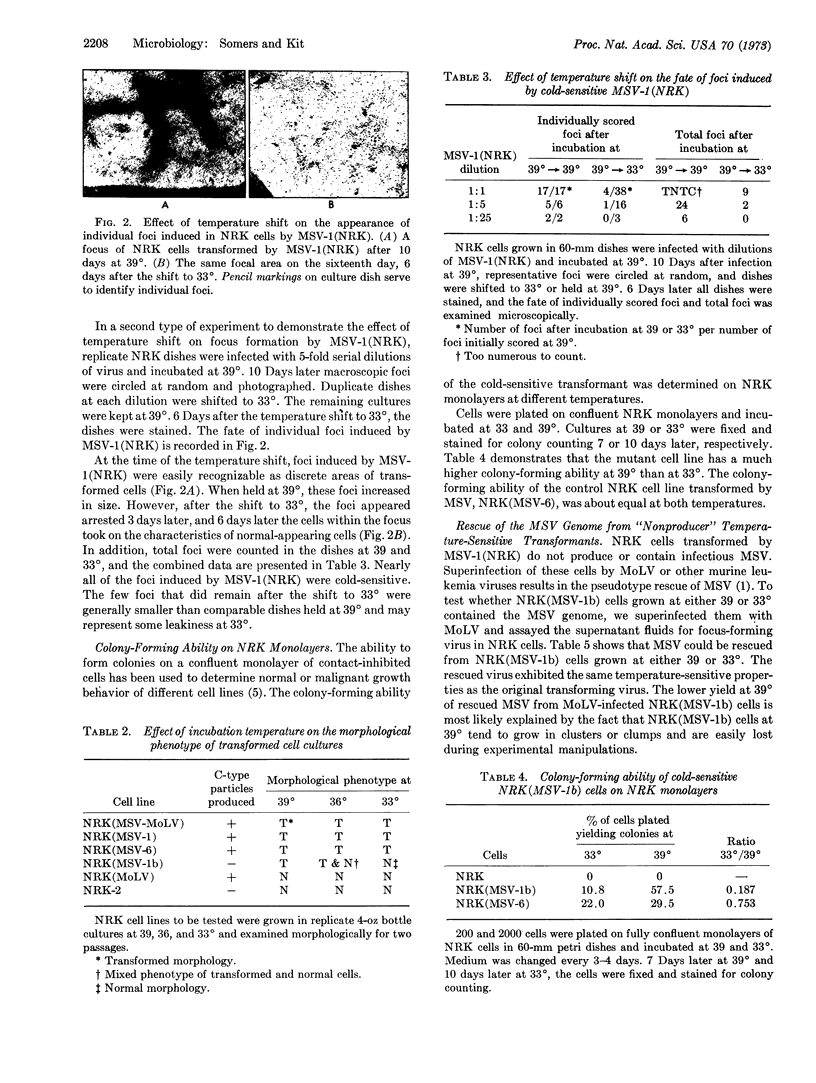


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Bassin R. H., Weaver C. Comparison of murine sarcoma viruses in nonproducer and S + L - -transformed cells. J Virol. 1972 Apr;9(4):701–704. doi: 10.1128/jvi.9.4.701-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Freeman A. E. Human sarcoma cells in culture. Identification by colony-forming ability on monolayers of normal cells. Exp Cell Res. 1970 Jul;61(1):1–5. doi: 10.1016/0014-4827(70)90250-8. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bayliss F. T., Vinopal R. T. Selection of ribosomal mutants by antibiotic suppression in yeast. Science. 1971 Dec 24;174(4016):1339–1341. doi: 10.1126/science.174.4016.1339. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Isolement et étude d'un mutant conditionnel du virus de Rous à capacité transformante thermosensible. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2430–2433. [PubMed] [Google Scholar]
- Christensen J. R., Saul S. H. A cold-sensitive mutant of bacteriophage T1. Virology. 1966 Jul;29(3):497–499. doi: 10.1016/0042-6822(66)90228-5. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Strack H. B. Cold-sensitive mutants of bacteriophage lambda. Genetics. 1971 Jan;67(1):5–17. doi: 10.1093/genetics/67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell C. E. Cold-sensitive mutants of bacteriophage phi-X-174. I. A mutant blocked in the eclipse function at low temperature. Proc Natl Acad Sci U S A. 1967 Sep;58(3):958–961. doi: 10.1073/pnas.58.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Peebles P. T., Haapala D. K., Bassin R. H. Reversion of murine sarcoma virus transformed mouse cells: variants without a rescuable sarcoma virus. Science. 1972 Jun 2;176(4038):1033–1035. doi: 10.1126/science.176.4038.1033. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- May J. T., Somers K. D., Kit S. Defective mouse sarcoma virus deficient in DNA polymerase activity. J Gen Virol. 1972 Aug;16(2):223–226. doi: 10.1099/0022-1317-16-2-223. [DOI] [PubMed] [Google Scholar]
- May J. T., Somers K. D., Kit S. Temperature-dependent alterations in 2-deoxyglucose uptake in rat cells transformed by a cold-sensitive murine sarcoma virus mutant. Int J Cancer. 1973 Mar 15;11(2):377–384. doi: 10.1002/ijc.2910110215. [DOI] [PubMed] [Google Scholar]
- Nomura S., Bassin R. H., Turner W., Haapala D. K., Fischinger P. J. Ultraviolet inactivation of Moloney leukaemia virus: relative target size required for virus replication and rescue of 'defective' murine sarcoma virus. J Gen Virol. 1972 Feb;14(2):213–217. doi: 10.1099/0022-1317-14-2-213. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Ingraham J. L. Cold-sensitive mutants of Escherichia coli resulting from increased feedback inhibition. Proc Natl Acad Sci U S A. 1965 Aug;54(2):451–457. doi: 10.1073/pnas.54.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Bassin R. H., Haapala D. K., Phillips L. A., Nomura S., Fischinger P. J. Rescue of murine sarcoma virus from a sarcoma-positive leukemia-negative cell line: requirement for replicating leukemia virus. J Virol. 1971 Nov;8(5):690–694. doi: 10.1128/jvi.8.5.690-694.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. Isolation of cold sensitive-rifampicin resistant RNA polymerase mutants of Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):737–744. doi: 10.1016/s0006-291x(71)80145-6. [DOI] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scotti P. D. A new class of temperature conditional lethal mutants of bacteriophage T4D. Mutat Res. 1968 Jul-Aug;6(1):1–14. doi: 10.1016/0027-5107(68)90098-5. [DOI] [PubMed] [Google Scholar]
- Somers K. D., Kirsten W. H. Focus formation by murine sarcoma virus: enhancement by DEAE-dextran. Virology. 1968 Sep;36(1):155–157. doi: 10.1016/0042-6822(68)90129-3. [DOI] [PubMed] [Google Scholar]
- Somers K., Kit S. Clonal isolation of murine sarcoma virus (MSV): characterization of virus produced from transformed cells. Virology. 1971 Dec;46(3):774–785. doi: 10.1016/0042-6822(71)90079-1. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Isolation of temperature-sensitive mutants of murine leukemia virus. Virology. 1972 Jun;48(3):749–756. doi: 10.1016/0042-6822(72)90158-4. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]





