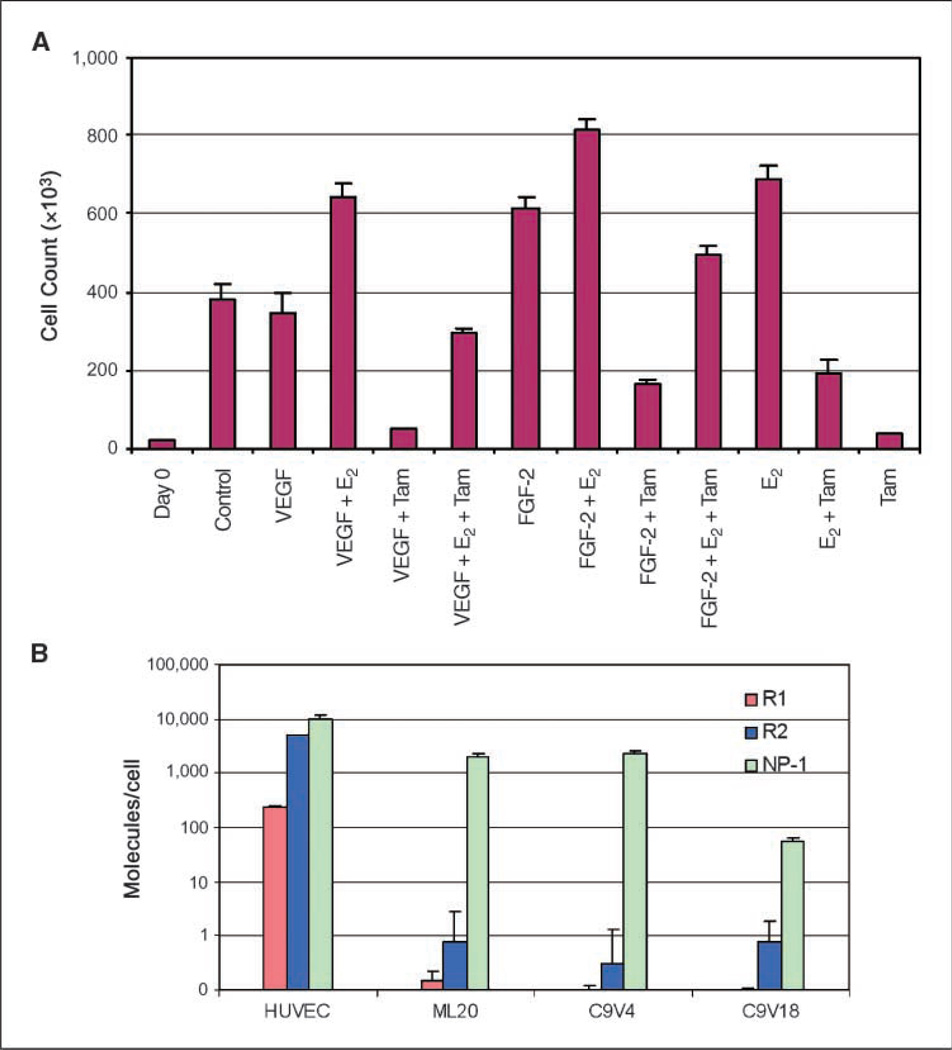
Figure 4.
Exogenous VEGF does not affect the sensitivity of C9V18 cells to estrogen or tamoxifen in vitro presumably due to the lack of VEGF tyrosine kinase receptor expression. A, in vitro cell proliferation assay. C9V18 cells were plated at a density of 104 per well in 24-well plates in 10% FBS IMEM. After 24 h, the culture media were exchanged with phenol red–free IMEM containing 0.1% charcoal-stripped calf serum to remove the estrogens overnight. Then, three wells were counted on day 0, and the indicated reagents in 1% charcoal-stripped calf serum, phenol red–free IMEM were added to the cultures in triplicate. The concentrations used were VEGF, 20 ng/mL; FGF-2, 20 ng/mL; 4-hydroxytamoxifen, 10−7 mol/L; and E2, 10−8 mol/L. The media were exchanged with the same conditions on day 4 and the cell numbers were counted with a Coulter counter on day 7. B, real-time quantitative RT-PCR analysis. Expression of VEGF receptors R1, R2, and NP-1 was examined with quantitative RT-PCR as described in Materials and Methods. The number of cells in each culture used for mRNA isolation was determined to allow calculation of the specific number of mRNA molecules per cell. mRNAs of the receptors R1 and R2 were determined to be <1 molecule per cell in MCF-7 and C9V cells, whereas about 200 R1 mRNAs and 5,000 R2 mRNAs are expressed per cell in human umbilical vein endothelial cells (HUVEC).