Abstract
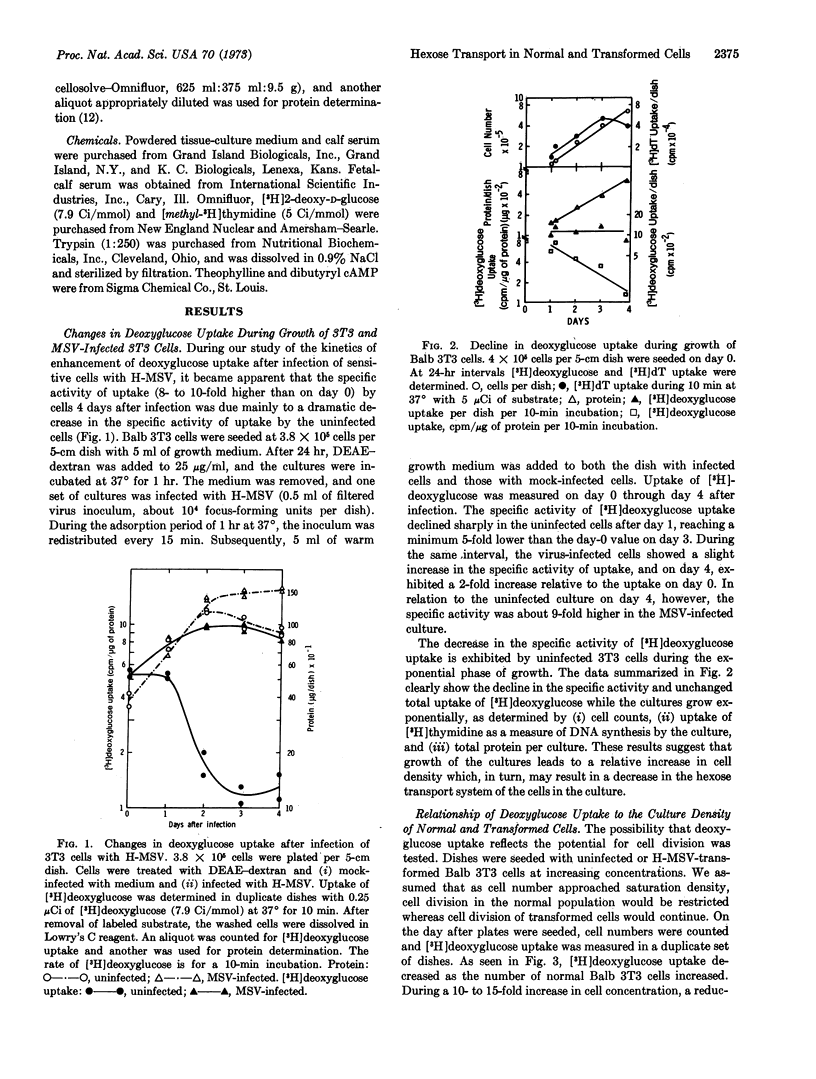
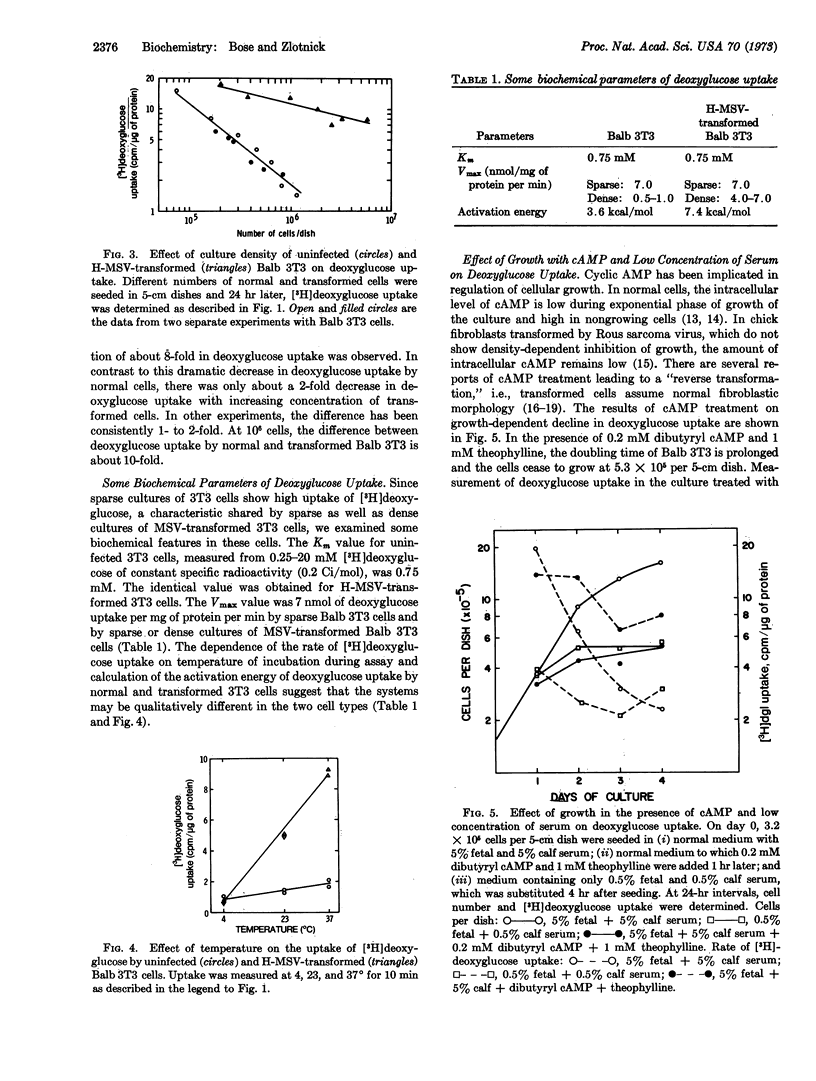
The earliest measurable parameter that shows density-dependent inhibition is the uptake of [3H]deoxyglucose by Balb 3T3 cells. The rate decreases even during the exponential phase of growth and reaches a minimum, about 8- to 10-times lower than maximum, as the culture approaches the saturation density. Cells transformed by murine sarcoma virus fail to show either growth-dependent or density-dependent inhibition of deoxyglucose uptake. Treatment with dibutyryl cyclic AMP in the presence of theophylline results in premature cessation of growth and in an arrest in the decline of deoxyglucose transport. Culture in serum-deficient medium also produces rapid inhibition of growth at low cell density, but these cultures exhibit a markedly decreased rate of deoxyglucose uptake.
Keywords: cell growth, cyclic AMP, low serum
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gazdar A., Hatanaka M., Herberman R., Russell E., Ikawa Y. Effects of dibutyryl cyclic adenosine phosphate plus theophylline on murine sarcoma virus transformed non-producer cells. Proc Soc Exp Biol Med. 1972 Dec;141(3):1044–1050. doi: 10.3181/00379727-141-36930. [DOI] [PubMed] [Google Scholar]
- Griffiths J. B. The effect of cell population density on nutrient uptake and cell metabolism: a comparative study of human diploid and heteroploid cell lines. J Cell Sci. 1972 Mar;10(2):515–524. doi: 10.1242/jcs.10.2.515. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Augl C., Gilden R. V. Evidence for a functional change in the plasma membrane of murine sarcoma virus-infected mouse embryo cells. Transport and transport-associated phosphorylation of 14C-2-deoxy-D-glucose. J Biol Chem. 1970 Feb 25;245(4):714–717. [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Paul D., Lipton A., Klinger I. Serum factor requirements of normal and simian virus 40-transformed 3T3 mouse fibroplasts. Proc Natl Acad Sci U S A. 1971 Mar;68(3):645–652. doi: 10.1073/pnas.68.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Stimulation of glucose transport in cultures of density-inhibited chick embryo cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3154–3157. doi: 10.1073/pnas.68.12.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W., Paul D. Levels of cyclic AMP in sparse and dense cultures of growing and quiescent 3T3 cells. Nat New Biol. 1972 Dec 27;240(104):281–283. doi: 10.1038/newbio240281a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Carcinogenesis by avian sarcoma viruses. X. The decreased requirement for insulin-replaceable activity in serum for cell multiplication. Int J Cancer. 1968 Nov 15;3(6):771–787. doi: 10.1002/ijc.2910030611. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Rucoslahti E., Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972 Aug 16;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Venuta S., Rubin H. Sugar transport in normal and Rous sarcoma virus-transformed chick-embryo fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):653–657. doi: 10.1073/pnas.70.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]


