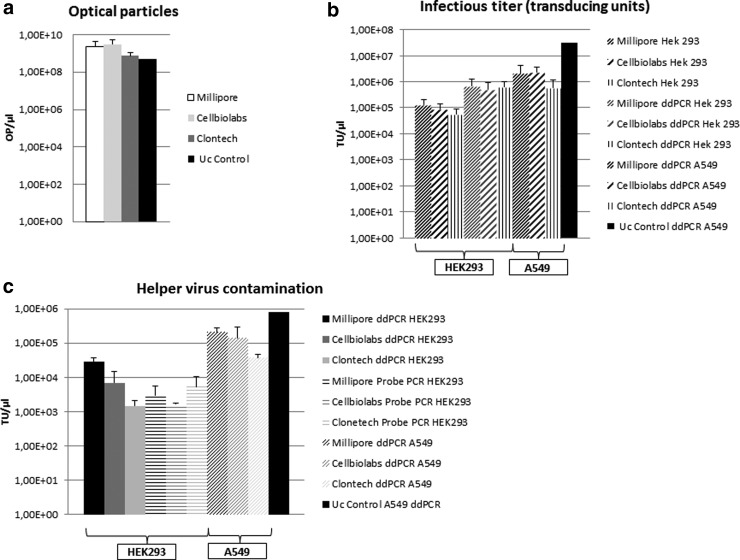
FIG. 4.
Evaluation of final HCAdV vector preparations. Cell lines in which vector titration was performed (HEK293 cells or A549 cells) and respective companies from which the adenovirus purification kits were obtained are indicated. (a) Physical titers expressed as optical particles measured with a photometer after different purification protocols. A differentiation regarding infectious particles or noninfectious particles is not possible using this approach. OP, optical particles. (b) Measurement of the infectious titer expressed as transducing units (TUs) using primer pair I (qPCR) and III (ddPCR). The diagram shows HCAdV detection in DNA samples collected from different cell lines 3 hr after treatment with the final purified vector preparations using traditional qPCR and ddPCR. For the data shown, different dilutions of the respective purified virus were used and the mean TU was calculated. The cell line in which the titration experiment was performed is indicated. (c) TUs of the HV (primer pair II for qPCR and primer pair IV for ddPCR) within the same preparations. For the ddPCR we acquired both datasets (HCAdV and HV) within one ddPCR run. Uc control, HCAdV purified by ultracentrifugation.