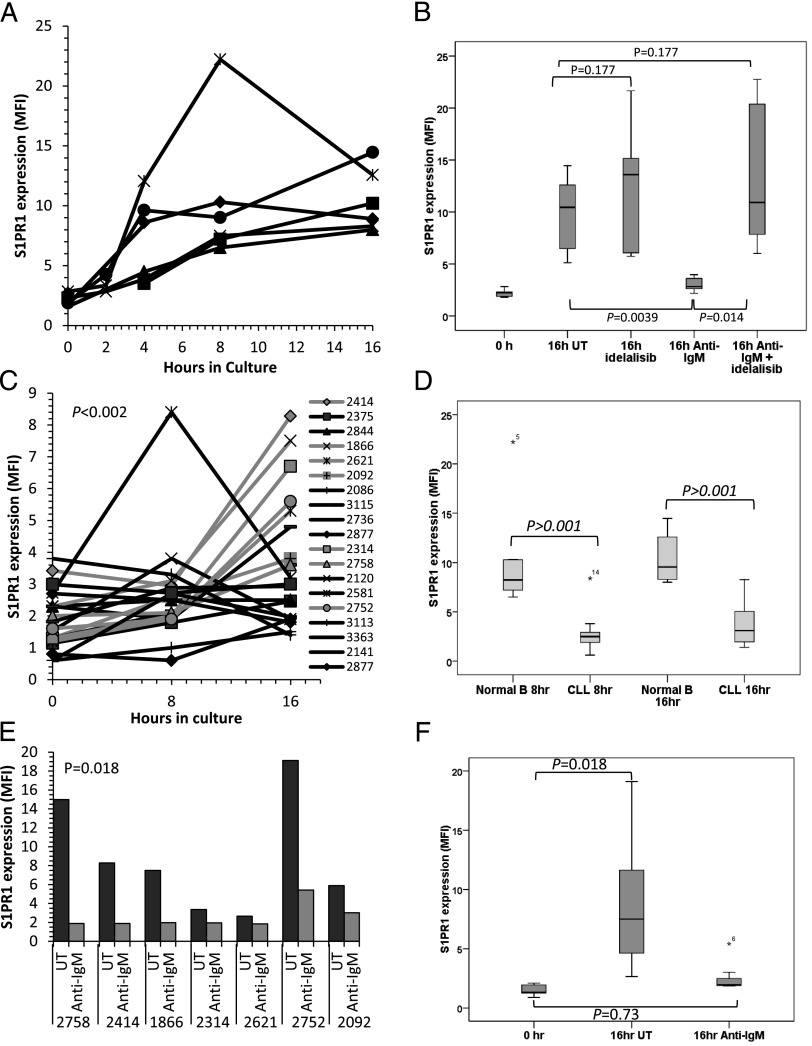
FIGURE 2.
S1PR1 expression on normal and CLL B cells cultured in the absence of S1P. (A) Normal B cells from six healthy individuals were cultured for 16 h in medium lacking S1P and examined for S1PR1 expression by flow cytometry. An increase in S1PR1 was observed between 2 and 4 h and reached a peak at 8–16 h. (B) Normal B cells from six donors were cultured in S1P-free medium for 16 h in the presence or absence of anti-IgM and/or idelalisib (1 μM). S1PR1 expression was measured by flow cytometry. IgM cross-linking prevented the spontaneous increase in S1PR1 expression (p = 0.0039). Idelalisib had little effect on S1PR1 expression on normal B cells in the absence of IgM (p = 0.177); however, treatment reversed the anti-IgM–mediated suppression of S1PR1 expression (p = 0.014) with levels returning to those of untreated cells at 16 h (p = 0.177). (C) CLL cells from 20 patients were cultured for 16 h in S1P-free medium and examined for S1PR1 levels by flow cytometry. There was an overall increase in S1PR1 expression (p < 0.002), but the increase was variable, delayed, and generally of lower magnitude compared with that observed in normal B cells. (D) Comparison of S1PR1 upregulation in normal and CLL B cells at 8 and 16 h. The increase in expression was significantly greater in normal B cells at both time points. In the box-and-whiskers plot, the bar indicates the median of the mean fluorescence intensity values for S1PR1 expression, whereas an asterisk (*) identifies outlying data points that do not fall within the interquartile range. (E) Seven of the CLL samples showing most spontaneous increase in S1PR1 expression [highlighted in gray in (C)] were cultured in S1P-free medium in the presence or absence of anti-IgM and examined for S1PR1 expression by flow cytometry. Upregulation of S1PR1 was prevented by BCR cross-linking in all cases. (F) Pooled analysis of the seven cases of CLL described in (E) showing near-complete abrogation of spontaneous upregulation of S1PR1 expression (p = 0.73).