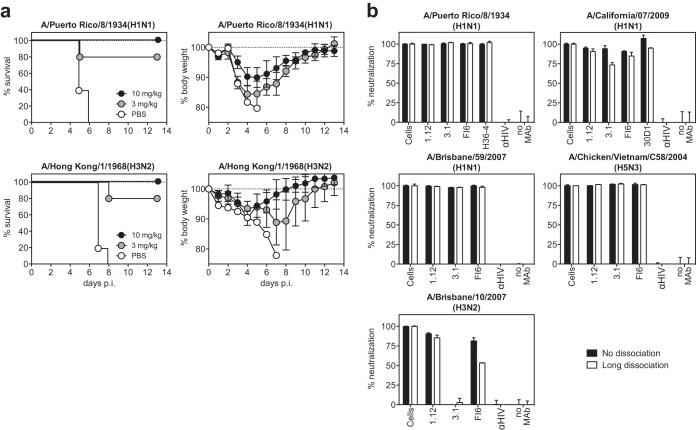
FIG 2.
Protection of mice and reversibility of neutralization. (a) Passive immunization of mice. Animals were injected intraperitoneally with the indicated dose of hMAb 1.12 in PBS or with PBS alone (control group) 24 h prior to intranasal infection with a lethal dose of A/Puerto Rico/8/34(H1N1) or mouse-adapted A/Hong Kong/1/1968(H3N2). Mice that dropped below 80 or 85% of their initial body weight were scored as dead and euthanized. For A/Puerto Rico/8/1934(H1N1), pooled data from two separate experiments are shown, each performed with 5 C57BL/6 females per group. One experiment was performed with 15% and one with 20% weight loss as the abortion criteria. For mouse-adapted A/Hong Kong/1/1968(H3N2), only the depicted experiment with a 20% weight loss abortion criterion was performed. (b) Reversibility of neutralization. Three HA stem-reactive antibodies were incubated at a concentration of 10 μg/ml with A/Puerto Rico/8/1934(H1N1), A/California/07/2009(H1N1), A/Brisbane/59/2007(H1N1), A/Brisbane/10/2007(H3N2), or A/Chicken/Vietnam/C58/2004(H5N3) virus (amounts corresponding to an MOI of ∼ 30) before the MAb-virus mixture was captured on magnetic beads. The beads were then processed so that no-dissociation and long-term-dissociation conditions were applied. In the last step, the residual infectivity of each sample was measured on MDCK cells. The HIV-1 gp120-specific MAb b12 was used as a negative control (αHIV). Uninfected and infected cells were included as positive and negative controls, respectively. Representatives of at least 2 consistent experiments (performed in duplicate) are shown. The error bars indicate standard deviations.