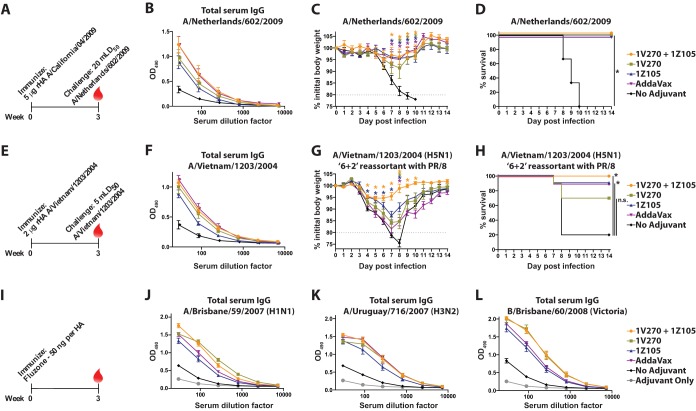
FIG 6.
1Z105 and 1V270 induce rapid protective immunity to the pH1N1 virus and an avian H5N1 subtype virus and enhance the immunogenicity of Fluzone. (A) BALB/c mice (n = 3/group) were immunized with rHA (5 μg/animal) derived from the pH1N1 virus (A/California/04/2009) on day 0 and bled 3 weeks later (red drop). (B) Total serum IgG titers were assayed by ELISA 3 weeks after immunization. (C and D) Three weeks after immunization, the mice were administered 5 mLD50 of a mouse-adapted pH1N1 virus (A/Netherlands/602/2009), which is essentially homologous to Cal/09, and monitored for morbidity (C) and mortality (D) induced by the challenge. (E) BALB/c mice (n = 10/group) were immunized with rHA derived from a highly pathogenic avian H5N1 virus (A/Vietnam/1203/2004) (2 μg/animal) on day 0. (F) Total serum IgG titers were assayed by ELISA 3 weeks after immunization. (G and H) Three weeks after a single immunization, the mice were challenged with 5 mLD50 of a 6-plus-2 reassortant of the VN/04 HA and NA (H5N1) in the PR/8 background and monitored for morbidity (G) and mortality (H) induced by the challenge. The data for the H5N1 challenge were combined from two experiments for a total of 10 mice/group. (I) BALB/c mice (n = 10/group) were immunized with 50 ng/HA of 2009–2010 Fluzone. (J to L) Serum IgG levels 3 weeks after immunization to each of the homologous viral components of the vaccine, including A/Brisbane/59/2007 (H1N1) (J), A/Uruguay/716/2007 (H3N2) (K), and B/Brisbane/60/2008 (Victoria lineage) (L), were assayed by ELISA. “No adjuvant” indicates animals that received the antigen in the absence of adjuvant, and mice in the vehicle group received an injection of 10% DMSO in PBS without antigen or adjuvant. The data shown are means and SEM. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; *, P < 0.05, which was considered significant.