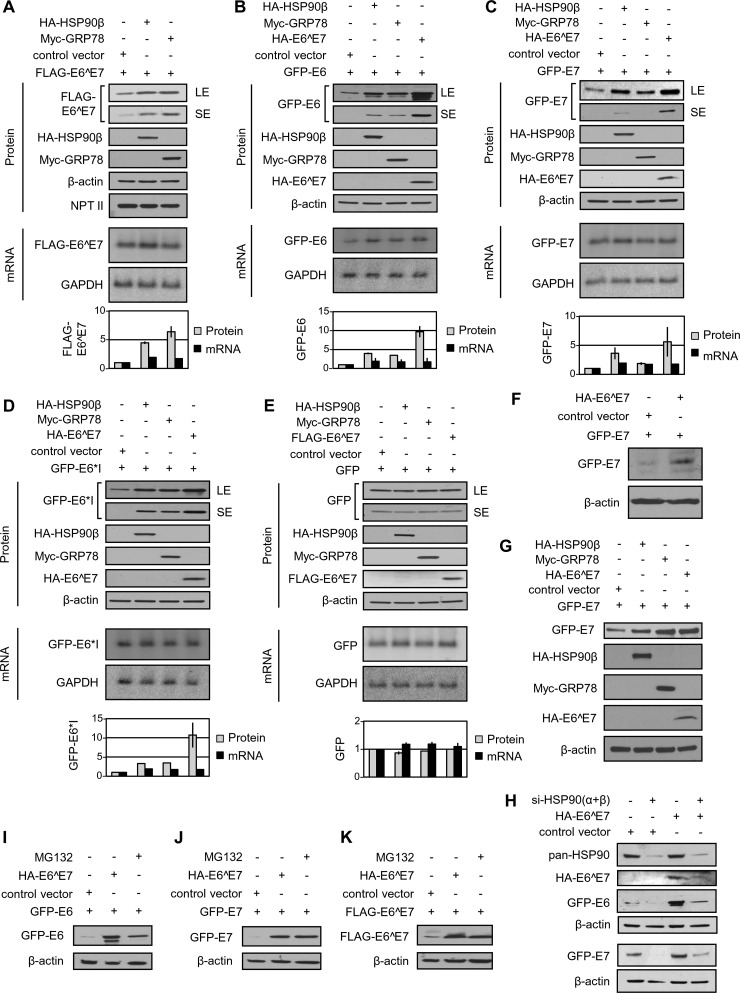
FIG 2 .
HPV16 E6^E7, HSP90, and GRP78 promote the protein stability of HPV16 E6 and E7. (A to E) ΗSP90β and GRP78 increase the protein but not mRNA levels of HPV16 E6^E7, E6, and E7 in HEK293 cells. Following cotransfection with the indicated vectors in each panel for 48 h, HEK293 cells were analyzed by Western blotting for protein expression levels of FLAG-E6^E7 (A), GFP-E6 (B), GFP-E7 (C), GFP-E6*I (D), or GFP (E) and by Northern blotting for the expression levels of corresponding mRNAs. An empty vector without any tagged protein expression served as a control in each transfection, and neomycin phosphotransferase II (NPT II, a neomycin resistance gene product) served as a control for plasmid transfection and expression efficiency. LE, longer exposure; SE, shorter exposure. After normalization with β-actin in a Western blot or with GAPDH in a Northern blot, the relative (fold) changes in the levels of the corresponding protein or mRNA in HEK293 cells cotransfected with HA-HSP90β, Myc-GRP78, or HA-E6^E7 over the levels in cells transfected with an empty control vector are shown at the bottom of each panel in bar graphs. Error bars indicate standard deviations from two different blots. (F) E6^7 increases the E7 protein level in primary human foreskin keratinocytes. The keratinocytes were cotransfected with 4D-Nucleofector and GFP-E6 plus an HA-E6^E7 expression vector or an empty control vector for 48 h and analyzed by Western blotting. (G) HA-HSP90β, Myc-GRP78, and HA-E6^E7 increase GFP-E7 protein level in HeLa cells. HeLa cells were cotransfected with the indicated vectors for 48 h and analyzed by Western blotting. (H) The effect of HPV16 E6^E7 on E6 and E7 stability relies on HSP90. HEK293 cells were transfected twice, with a 48-h interval, with siRNAs specific for HSP90α and -β isoforms or a nontargeting control siRNA (−) for 4 days. During the second siRNA transfection, the cells were cotransfected with an HA-E6^E7 expression vector or a control vector (p3×FLAG-CMV14) in combination with a GFP-E6 or -E7 expression vector for 24 h and analyzed by Western blotting for HSP90α and -β knockdown efficiency with a pan-HSP90 antibody, E6^E7 with an anti-HA antibody, or HPV16 E6 or E7 with an anti-GFP antibody. β-actin served as a loading control. (I to K) HA-E6^E7 increased protein levels, and the levels of MG132-stabilized GFP-E6, GFP-E7, and FLAG-E6^E7 are comparable in HEK293 cells at 48 h of transfection. The cell cotransfections were conducted with a GFP-E6 (I), GFP-E7 (J), or FLAG-E6^E7 (K) expression vector along with an HA-E6^E7 expression vector or control vector. For MG132 treatment, the cells transfected with a GFP-E6, GFP-E7, or FLAG-E6^E7 expression vector were treated with MG132 (10 µM) or an equivalent amount of dimethyl sulfoxide (DMSO) 6 h prior to being harvested for Western blotting with an anti-GFP or anti-FLAG antibody. β-actin served as a sample loading control.