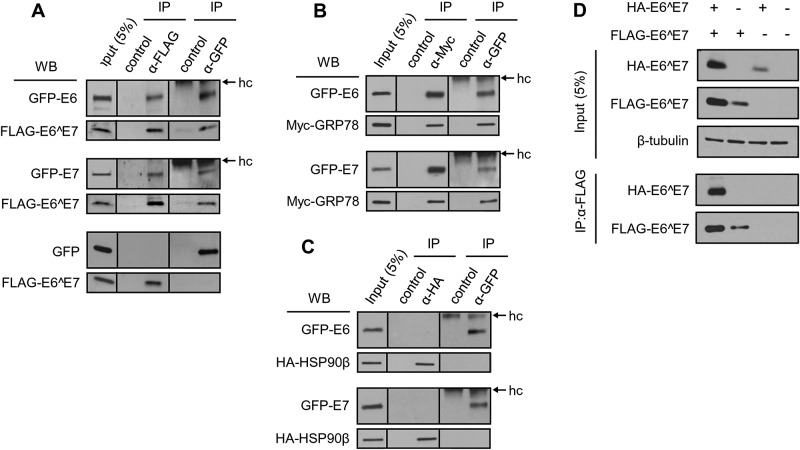
FIG 3 .
HPV16 E6 and E7 oncoproteins are proteins that interact with E6^E7 and GRP78. (A to C) HPV16 E6 and E7 interact with E6^E7 (A) and GRP78 (B), but not with HSP90β(C). HEK293 cells were transfected with FLAG-E6^E7 (A), Myc-GRP78 (B), or HA-HSP90β (C) along with GFP-E6 or -E7 (A to C) for 48 h. The cell lysates were immunoprecipitated with the corresponding antibody, as indicated. Rabbit IgG was used as a negative control for each anti-GFP IP, and Sepharose beads without antibody served as controls for anti-FLAG, anti-HA, and anti-Myc IP. The interacting proteins in coimmunoprecipitations or in the input were examined by Western blotting (WB) with anti-GFP for GFP-E6 or -E7 (A to C), anti-FLAG for FLAG-E6^E7 (A), anti-Myc for Myc-GRP78 (B), or anti-HA antibody for HA-HSP90β(C). hc, IgG heavy chain. (D) E6^E7 is a self-interacting protein. HEK293 cells were cotransfected with a FLAG-E6^E7 and an HA-E6^E7 expression vector or an empty (−) control vector for 48 h. The cell lysates were blotted for the expression level of each protein (input panel) and immunoprecipitated with anti-FLAG antibody. The proteins pulled down by IP were blotted with an anti-FLAG antibody for FLAG-E6^E7 or anti-HA antibody for HA-E6^E7. β-Tubulin served as a sample loading control.