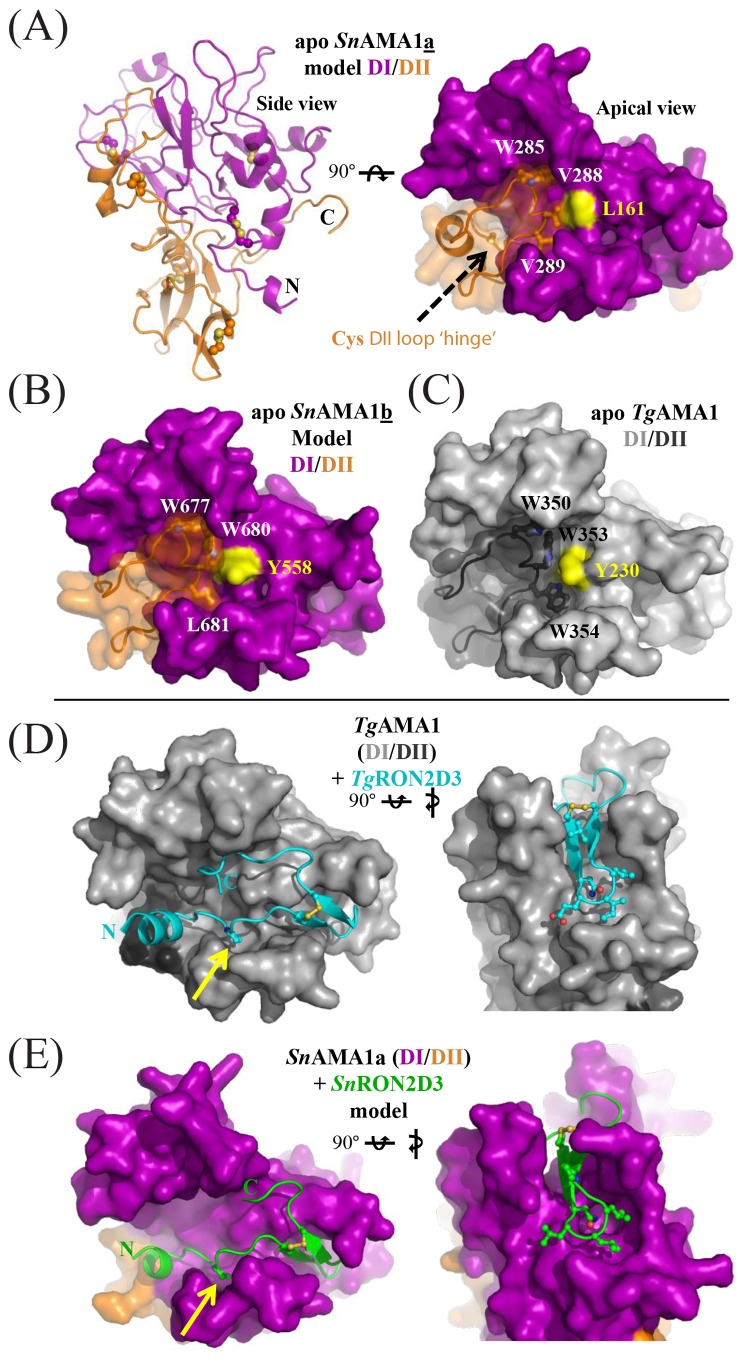
FIG 4 .
SnAMA1a and SnAMA1b accessorize the canonical AMA1 DI and DII domains with unique features but maintain an apical surface capable of coordinating SnRON2D3. (A) Secondary-structure (left) and surface representations of SnAMA1a DI (purple) and DII (orange); five conserved disulfides and two extra cysteines in the DII loop are highlighted as ball-and-stick structures. Two cysteines predicted to form a disulfide at the DII loop hinge are shown as a ball-and-stick structure (black arrow). Residues anchoring the DII loop are labeled and surround a central Leu residue colored yellow. (B) Surface representations of SnAMA1b colored and labeled as for SnAMA1a. (C) Surface representation of TgAMA1 (Protein Data Bank [PDB] accession no. 2x2z); DI, light grey; DII, dark grey. (D) Complementary views of the TgAMA1-TgRON2sp costructure (PDB accession no. 2y8t) with TgAMA1 colored light grey (DI) or dark grey (DII) and TgRON2sp colored cyan. (E) Complementary views of the SnAMA1a-SnRON2D3 costructure model, with SnAMA1 colored as in panel A and SnRON2D3 in green. Residues making up the RON2 cystine loop tip are shown as ball-and-stick structures to highlight shape complementarity.