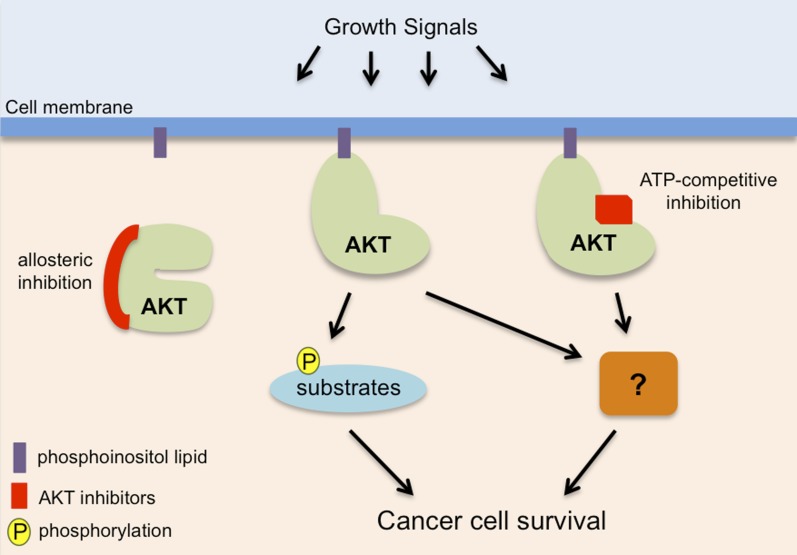
Figure 1. AKT, also known as protein kinase B, promotes cancer cell survival in two distinct ways.
AKT (pale green) is recruited to phosphoinositol lipids (purple) at the cell membrane. Normally it is only activated in response to growth or survival signals, but it has increased activity in many cancers. It has been known for some time that AKT promotes the survival of cancer cells by adding phosphate groups (yellow) to protein substrates (light blue): this process involves ATP (not shown) binding to an active site in the kinase domain of the AKT, so it can be inhibited by drugs that compete with ATP to bind to this site (red rectangle). Vivanco, Chen et al. show that AKT can also promote cancer cell survival in a way that is independent of its kinase function: however, the details of this process remain unclear (hence the question mark). Drugs that compete with ATP do not inhibit this kinase-independent role, but allosteric inhibitors (left; see main text) inhibit both the kinase-dependent and kinase-independent roles of AKT, so they have the potential to be more effective therapies to treat cancer.