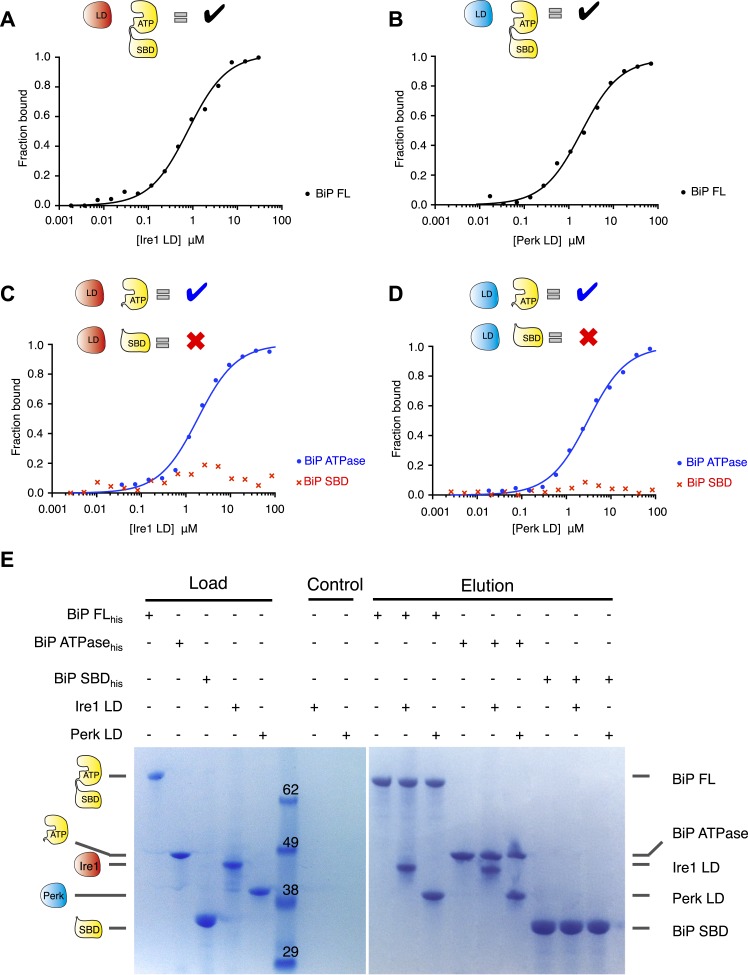
Figure 1. Noncanonical binding of BiP ATPase domain to Ire1 and Perk.
(A–B) Microscale thermophoresis (MST) analysis showing sigmoidal binding curves for interaction between full-length BiP and the complete luminal domains (region I–V) of (A) Ire1 luminal domain (Kd = 1.33 μM) and (B) Perk luminal domain (Kd = 1.92 μM). (C–D) MST binding curves of interaction between BiP sub-domains (ATPase and substrate binding domain) and (C) Ire1 luminal domain (ATPase Kd = 1.97 μM; no binding to substrate binding domain) and (D) Perk luminal domain (ATPase Kd = 2.05 μM; no binding to substrate binding domain). (E) Pull down assay showing BiP-luminal domain complexes using His6-tagged BiP proteins and luminal domains of Perk and Ire1 visualized by coomassie brilliant blue stained SDS PAGE gel. Ire1 and Perk luminal domains bind to full-length BiP and BiP ATPase domain. No binding to BiP substrate binding domain was observed.