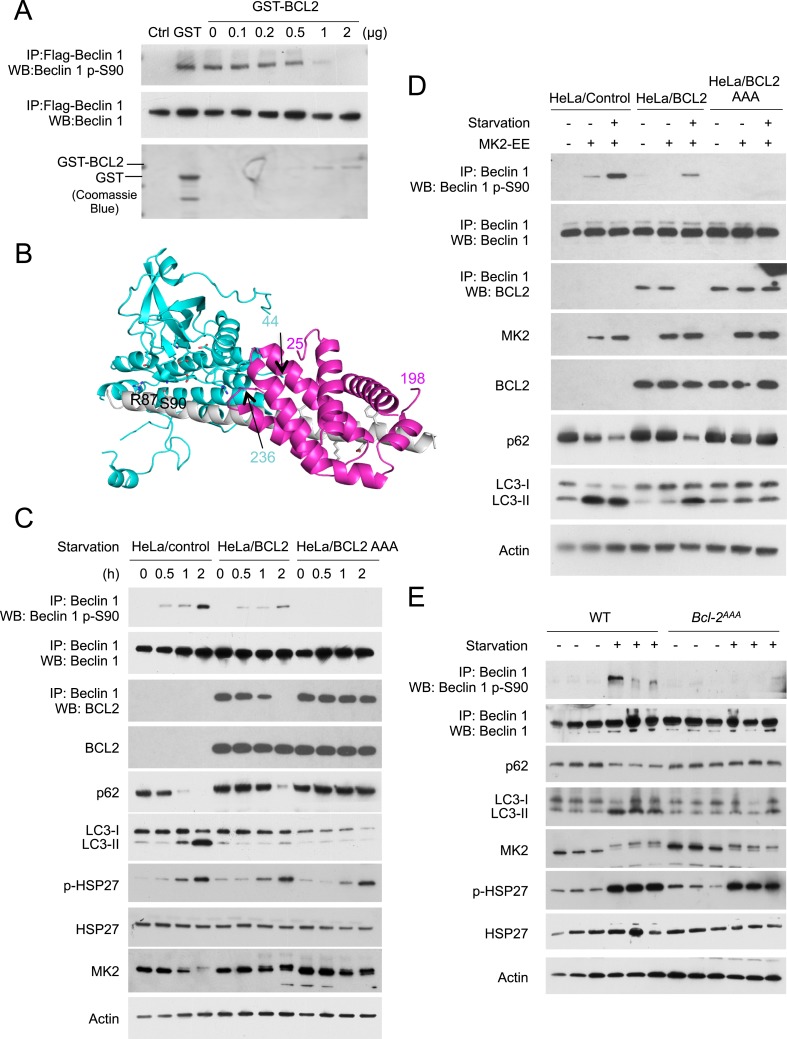
Figure 7. BCL2 inhibits MK2-dependent Beclin 1 S90 phosphorylation.
(A) In vitro kinase activity of MK2 using Flag-Beclin 1 purified from HEK293 cells as a substrate in the presence of the indicated amount of GST-BCL2. (B) Structural model showing that steric hindrance may prevent simultaneous binding of BCL2/BCL2L1 and MK2 to Beclin 1. MK2 (residues 44–216, 236–284 and 288–345), BCL2L1 (residues 2–25 and 83–198) and Beclin 1 (residues 79–181) are shown in cyan, pink and grey ribbon, respectively. MK2 residues important for catalysis and Beclin 1 residues important for binding to BCL2L1, or for phosphorylation (S90 and R87) are shown in stick, with atoms colored by element type: oxygen, red; nitrogen, blue; sulfur, green; and carbon, cyan or grey for MK2 or Beclin 1 respectively. Numbers indicate the last residue preceding or following regions missing from this model, that might cause further steric conflicts. (C) Detection of Beclin 1 S90 phosphorylation and autophagy levels (as measured by p62 and LC3 immunoblots) in HeLa cells stably transfected with empty vector (HeLa/control), wild-type BCL2 (HeLa/BCL2), or a BCL2 T69A/S70A/S87A mutant (HeLa/BCL2 AAA) and subjected to starvation in HBSS for the indicated time period. p-Hsp27 protein levels are shown as an indicator of MK2 activation. (D) Detection of Beclin 1 S90 phosphorylation and autophagy levels (as measured by p62 and LC3 immunoblots) in HeLa/Control, HeLa/BCL2, and HeLa/BCL2 AAA cells transiently transfected with a control empty vector or constitutively active MK2 (MK2 EE) and grown in normal medium (starvation−) or HBSS for 2 hr (starvation+). (E) Starvation-induced Beclin 1 S90 phosphorylation and autophagy induction in vastus lateralis muscle of Bcl2AAA mice and control wild-type littermates. Mice were subjected to starvation for 48 hr prior to tissue collection and western blot analysis of muscle lysates with indicated antibodies. Each lane represents a muscle sample from an independent mouse.