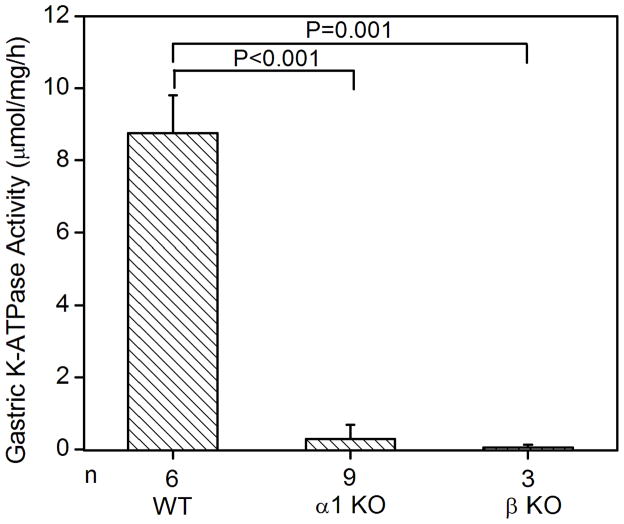
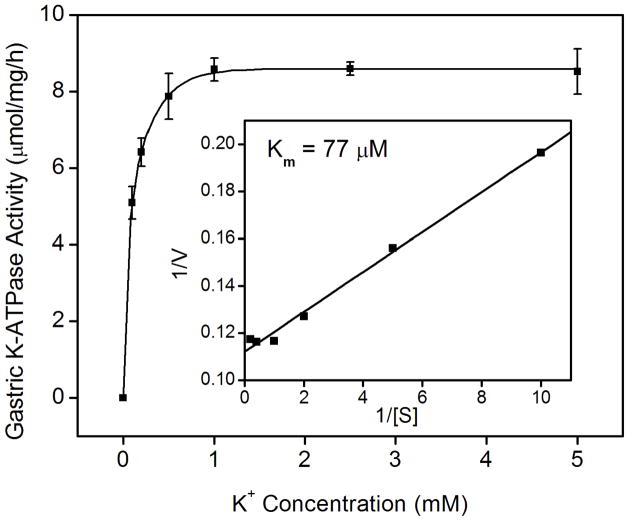
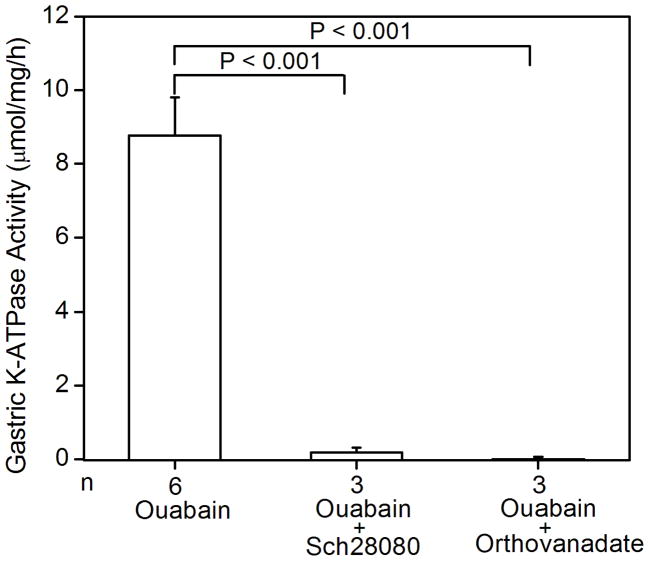
Figure 1.
K-ATPase activity in the gastric membrane vesicles of mice (A) Gastric K-ATPase activity was determined in the gastric membrane vesicles of wild type (WT), HKα1 knockout (α1 KO), and HKβ knockout (β KO) mice in the presence of 1 mM ouabain. Values (in micromole ATP hydrolyzed per mg membrane protein per hour) are means ± se from the indicated number of animals (n). (B) K-dependent ATPase activity was measured in the gastric membrane vesicles extracted from HKα1 wild type mice. K-ATPase activity of mouse gastric mucosa was fully stimulated by 1.0 mM K+. Inset, double-reciprocal plot of 1/V versus 1/[S] indicates apparent Km of 77 μM for the gastric K-ATPase activity. (C) Gastric K-ATPase activity was measured in the presence of 1 mM ouabain or in the presence of ouabain plus 100 μM Sch28080, or ouabain plus 100 μM orthovanadate. Values are means ± se from the indicated number of animals (n). Comparisons between groups were performed by Student’s t-test.