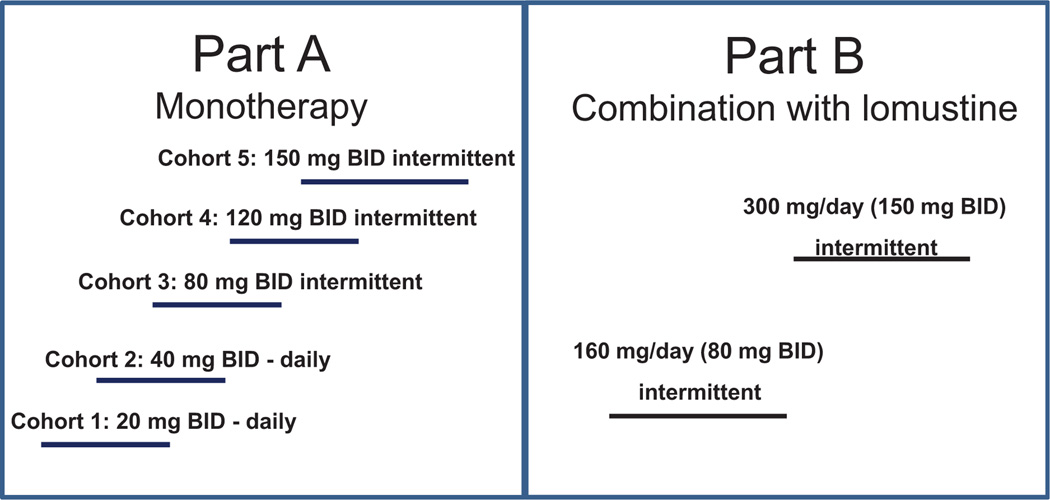
Figure 1.
FHD study design: part A, monotherapy and dose escalation. Cohorts 1 and 2 continuous dosing (twice daily, BID) and cohorts 3 to 5 of intermittent dosing (twice daily) on 14-day on and 14-day off cycle (28-day cycle). Part B, combination therapy with lomustine (100 to 130 mg/m2) with >3 patients in each dose group. The combination therapy in two cohorts at 160 mg/day (80 mg twice daily) and 300 mg/day (150 mg twice daily) with LY2157299 given intermittently.