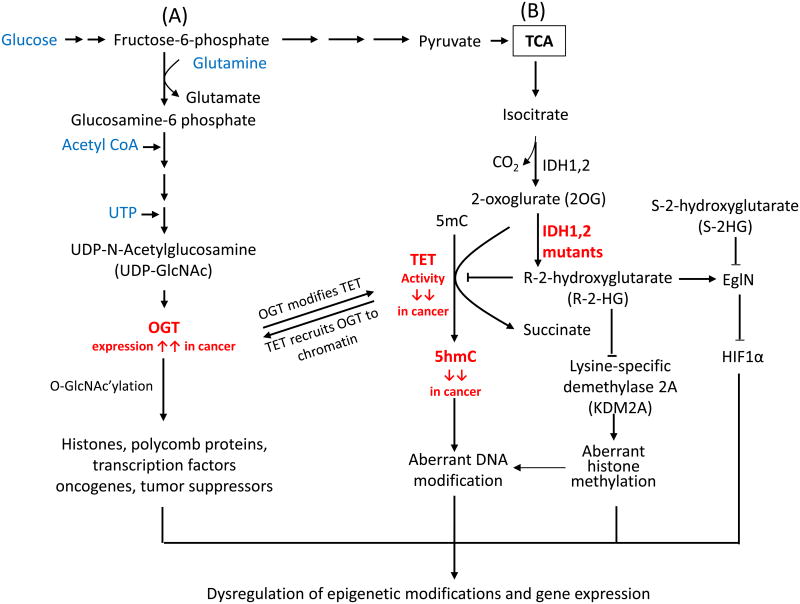
Figure 4. Crosstalk between TET and key enzymes involved in protein glycosylation and metabolism.
(A) Glucose metabolism modulates the activity of OGT. OGT (O-linked β-N-acetylglucosamine transferase) utilizes UDP-GlcNAc to glycosylate serine and threonine residues of diverse nuclear and cytoplasmic proteins, including enzymes involved in epigenetic regulation in cancer. TET proteins facilitate the O-GlcNAc modification of histones and chromatin remodeling enzymes by recruiting OGT to chromatin. Conversely, TET proteins are O-GlcNAc-modified by OGT, potentially increasing their stability and promoting TET3 nuclear localization in mESC or cultured cell lines. However, the stability of TET protein might also be regulated by other proteins, such caspase and calpain in cancer cells. (B) Isocitrate dehydrogenase (IDH) enzymes generate 2-oxoglurate (2OG), an essential co-factor for TET proteins. In contrast, mutant IDH enzymes bearing recurrent cancer-associated mutations deplete 2OG by converting it to 2-hydroxyglutarate (2HG), thus inhibiting TET enzymatic activity and decreasing the genomic levels of 5hmC. 2HG has two enantiomers, R-2HG and S-2HG. Mutant IDH enzymes only yield the oncometabolite R-2HG, which inhibits TET proteins and other dioxygenases, although it is typically less effective than S-2HG. R-2HG also promotes the enzymatic activity of EgIN prolyl-4-hydroxylases, enzymes that regulate the protein levels of the transcription factor HIF1a through hydroxylation followed by proteasomal degradation. By contrast, S-2HG can antagonize the proto-oncogenic activity induced by IDH mutants and EgIN.