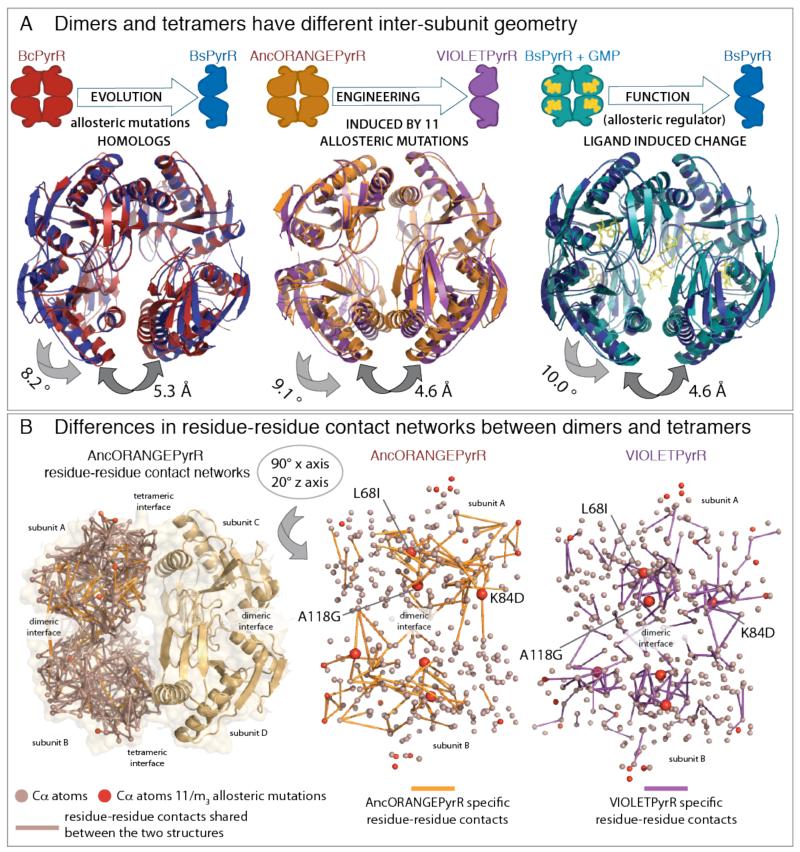
Fig. 4.
(A) Change in oligomeric state through evolutionary variation, functional allostery, or recapitulated by engineering is always coupled to the same difference in inter-subunit geometry. The three superimposed pairs of structures: (i) Bacillus subtilis PyrR (BsPyrR, pdb:1a3c) dimers superimposed on Bacillus caldolyticus PyrR (BcPyrR, pdb: 1non) tetramer; (ii) VIOLETPyrR dimers superimposed on AncORANGEPyrR tetramer; (iii) Bacillus subtilis PyrR (BsPyrR, pdb:1a3c) dimers superimposed on tetrameric Bacillus subtilis PyrR in complex with GMP. The inter-subunit geometry of free BsPyrR is incompatible with formation of the dihedral tetramer, however, GMP binding introduces a 10° inter-subunit geometric change and BsPyrR forms a tetramer. The VIOLETPyrR inter-subunit geometry is not compatible with the formation of the tetramer formed by AncORANGEpyrR, and this difference of conformation and oligomeric state is brought about by eleven allosteric mutations. (B) The tetrameric AncORANGEPyrR and dimeric VIOLETPyrR residue-residue contact networks differ by 15% of their contacts. Orientation of networks can be further explored in Supplementary Videos S1 and S2. L68I, K84D and A118G are central hubs and are involved in the majority of contact rewiring between the dimeric and tetrameric networks.