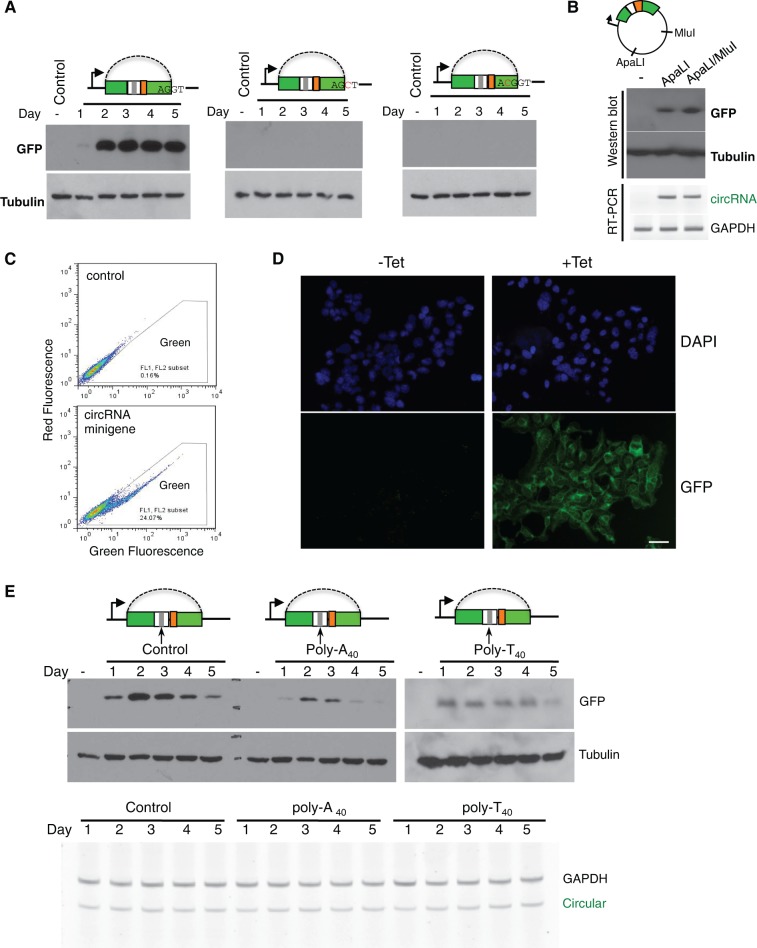
FIGURE 3.
In vivo translation directed by circular mRNA. (A) Cells were transfected with wild-type or mutated minigenes containing structured introns, and total proteins were purified at different days after transfection to detect production of GFP. One mutated minigenes has a disrupted 5′ splice site (second panel), while the other contains a C residue inserted into exon to disrupt GFP reading frame (third panel). (B) The circRNA minigene vector has single ApaLI and MluI cut sites in the plasmid backbone. We linearized the wild-type minigene with ApaLI or ApaL1/MluI digestion, and transfected the linearized DNA into 293 cells to detect GFP expression at 2 d after transfection. The production of circRNA was also measured by RT-PCR as described in Figure 1. (C) Cells transiently transfected with backsplicing minigene are assayed by flow cytometry at 2 d after transfection. (D) 293 FlpIn T-REX cells were stably transfected with backsplicing minigene containing structured intron, and the production of GFP was induced by addition of tetracycline at a final concentration of 1 μg/mL. The cells were assayed by fluorescence microscopy 48 h after induction. Scale bar = 50 μm. (E) Production of GFPs from minigenes inserted with poly(A40), poly(T40), or nonrepetitive control sequence downstream from the stop codon was detected with Western blot. The tubulin antibody was used as a loading control. The Western blot at the right panel (poly-T40) was overexposed to show protein production. Bottom, the corresponding RNA levels from each transfected sample were determined with semiquantitative RT-PCR.