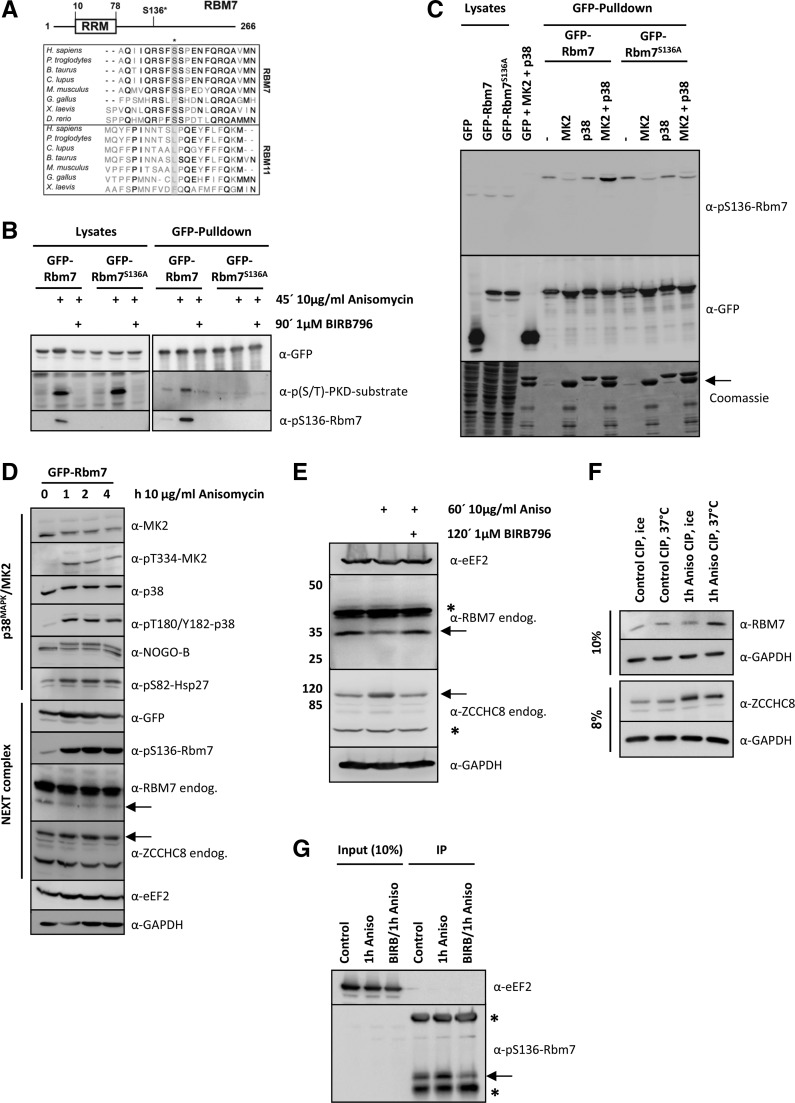
FIGURE 1.
RBM7 is phosphorylated by MK2 in vivo and in vitro. (A) Multiple sequence alignment of vertebrate RBM7 and its closest homolog RBM11. Displayed amino acid sequences span the human RBM7 S136 MK2 phosphorylation site (indicated by a star). The MK2-site is absent in RBM11. (B) Murine GFP-Rbm7 and GFP-Rbm7S136A were overexpressed in HeLa cells and treated as indicated. Upon precipitation with the help of a GFP-Nanotrap lysates and precipitates were developed with p(S/T)-PKD-substrate and pS136-Rbm7 antibodies to analyze Rbm7 phosphorylation and a GFP-antibody as loading control. Only wild-type Rbm7 gave rise to a specific phosphorylation on S136 upon anisomycin stimulation, whereas BIRB796-treatment and S136 to A mutation resulted in a loss of the phospho-signal. (C) Murine GFP-Rbm7 and GFP-Rbm7S136A were again overexpressed in HeLa cells and precipitated with a GFP-Nanotrap upon stimulation as indicated. The precipitates were then incubated with recombinant MK2 and p38 proteins for 30 min at 30°C to trigger in vitro phosphorylation. Afterward, samples were resolved by SDS-PAGE and either transferred to a nitrocellulose membrane and developed with the specific pS136-Rbm7- or GFP-antibody to monitor equal precipitation or stained by Coomassie. As demonstrated with the in vivo experiments (2B and 2C) only active MK2 (MK2 + p38) could phosphorylate Rbm7 in vitro. Precipitated GFP-Rbm7 and GFP-Rbm7S136A was detected at the same molecular weight as recombinant MK2. Its positions are indicated by an arrow. (D) Murine GFP-Rbm7 was expressed in HeLa cells and stimulated with anisomycin for 0, 1, 2, and 4 h to gain information on the phosphorylation kinetics of RBM7 and the p38MAPK-signaling pathway components and targets. The anti-pS136-Rbm7 antibody only detected the phosphorylated exogenous GFP-tagged Rbm7 protein. The positions of endogenous RBM7 and ZCCHC8 are indicated by arrows. Anisomycin stimulation altered the band intensities of RBM7 and ZCCHC8. (E) To analyze the potential phosphorylation of endogenous RBM7 and ZCCHC8 in more detail, extracts of HeLa cells treated as indicated were resolved on 8% and 12% SDS gels, respectively. Still, the endogenous RBM7-signal is weaker when cells are treated with anisomycin and the ZCCHC8 signal shifts slightly to a higher molecular weight. Both effects could point to a phosphorylation of the endogenous proteins. Unspecific bands coming up with antibodies detecting endogenous RBM7 and ZCCHC8 are marked with a star. (F) To verify endogenous RBM7 and ZCCHC8 phosphorylation, cell lysates that were treated as indicated and incubated with phosphatase (CIP, see Materials and Methods) were analyzed by Western blotting upon running 8% or 10% gels, respectively. (G) Endogenous RBM7 from HeLa cells that were treated as in F was immunoprecipitated using the anti-pS136-Rbm7 antibody (see Materials and Methods for details). Samples were analyzed for the presence of pS136-RBM7 using the same antibody and eEF2 for equal loading. The arrow indicates endogenous phosphorylated RBM7 and the stars indicate for the heavy and the light of the antibody used for IP.