Fig. 5.
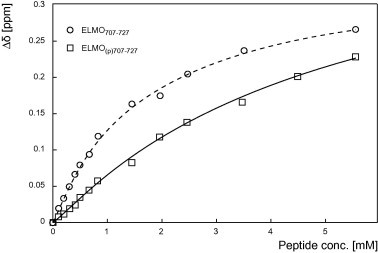
Comparison of the interaction between the SH3 domain of Hck and the phosphorylated or the non-phosphorylated polyproline motif of ELMO1. Titration of the 15N-labeled SH3 domain of Hck with increasing amounts of the phosphorylated ELMO(p)707–727 peptide (open squares) or of the non-phosphorylated ELMO707–727 peptide (open circles). The titration curves of the chemical shift variations of the representative tryptophan 113 side chain are compared as a function of the peptides concentrations (up to 55 equivalents). These variations were measured for at least five different residues and the dissociation constant was calculated as described in the Section 2.
