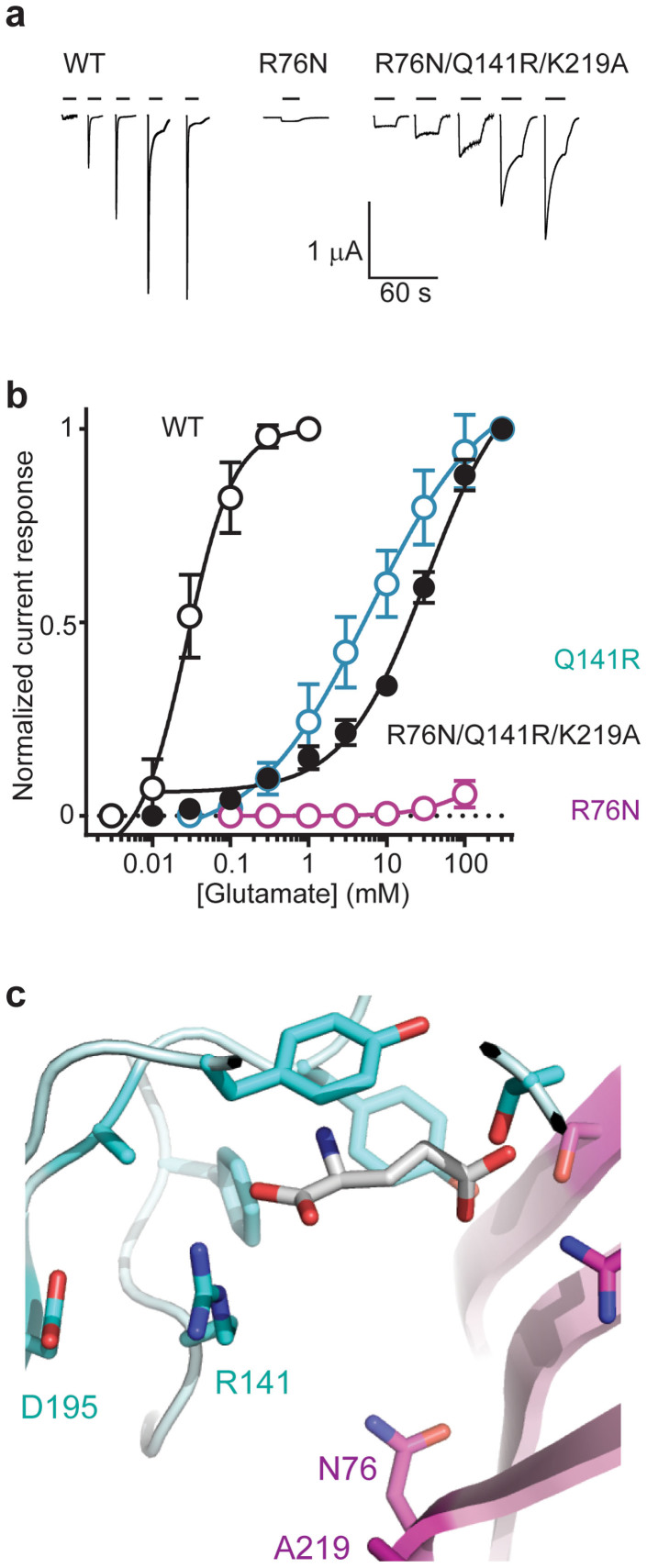
Figure 3. A principal or complementary subunit arginine recognizes the glutamate α-carboxyl in an ecdysozoan GluCl.
(a) Glutamate gates robust currents at WT and triple-mutant R76N/Q141R/K219A but not at single-mutant R76N GluCls. Current responses to glutamate are shown for oocytes injected with WT or mutant cRNAs, as indicated. Bars indicate glutamate application (0.01, 0.03, 0.1, 0.3 and 1 mM for WT; 100 mM for R76N; 1, 3, 10, 30 and 100 mM for R76N/Q141R/K219A). (Scale bars refer to all experiments.) (b) Averaged glutamate dose-response data. Mean (±s.e.m.) peak glutamate-gated currents were normalized to maximum glutamate-gated current (n = 4–5), except for R76N (n = 6), which was normalized to averaged ivermectin-gated current amplitude (reported in Table 1). Data are fit with non-linear regression for illustration. (c) Close-up of computational glutamate docking to triple-mutant R76N/Q141R/K219A AVR-14B GluCl model. R141 ε and η nitrogen atoms are 3.2 and 3.0 Å from one agonist α-carboxyl oxygen atom. Only residues at the bottom of the site are labeled, including D195, just prior to Loop B; other residues appear as in Fig. 2a.