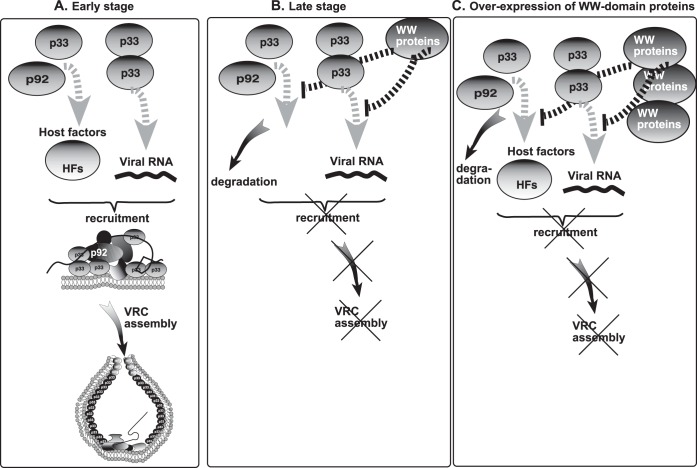
FIG 9.
Models for the role of WW-domain proteins in regulation of tombusvirus replication. We propose that WW-domain proteins inhibit the assembly of new VRCs but do not affect already-assembled VRCs. (A) At the early stage of replication, the tombusvirus replication proteins bind primarily to the abundant cellular susceptibility factors (proviral host factors [HFs]), to other viral replication proteins, and to the viral (+)RNA to recruit the viral (+)RNA to cellular membranes and assemble functional VRCs. Binding to WW-domain proteins by the replication proteins is inefficient under these conditions. (B) At the late stage of replication, the easily accessible host factors are depleted (scarce due to sequestration into previously assembled VRCs) and the new viral replication proteins bind to WW-domain proteins, leading to a blockage in new VRC assembly. (C) Overexpression of the WW-domain proteins facilitates binding to the viral replication proteins to WW-domain proteins (at the expense of interaction between replication proteins and host factors), leading to a blockage in VRC assembly and reduced level of TBSV replication. Thus, WW-domain proteins can also act as CIRFs.