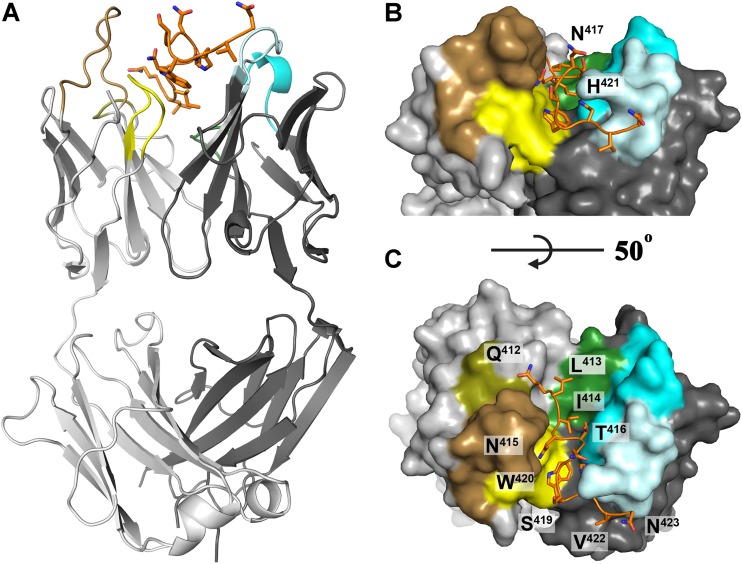
FIG 2.
Crystal structure of Fab 3/11 in complex with its peptide epitope. (A) The crystal structure of the Fab 3/11-peptide complex was determined and refined to a 2.2-Å resolution (shown as a cartoon). The peptide (orange) adopts an extended conformation and interacts with the Fab mainly in the cleft between the heavy (dark gray) and light (light gray) chains. Complementarity-determining regions 1, 2, and 3 are shown in cyan, light cyan, and dark green, respectively, for the heavy chain and in sand, olive, and yellow, respectively, for the light chain. The peptide N terminus interacts mostly with the light chain, and its C-terminal part makes a right-angle turn around complementarity-determining region 2 of the heavy chain (CDR-H2). C-terminal residues H421, V422, and N423 interact exclusively with the heavy chain. (B and C) View on the paratope of the Fab 3/11-peptide complex from two angles illustrating how deeply the peptide immerses into the cleft between the heavy and light chains. The molecular surface, the peptide, and the complementarity-determining regions of the Fab fragment are colored as described above for panel A.