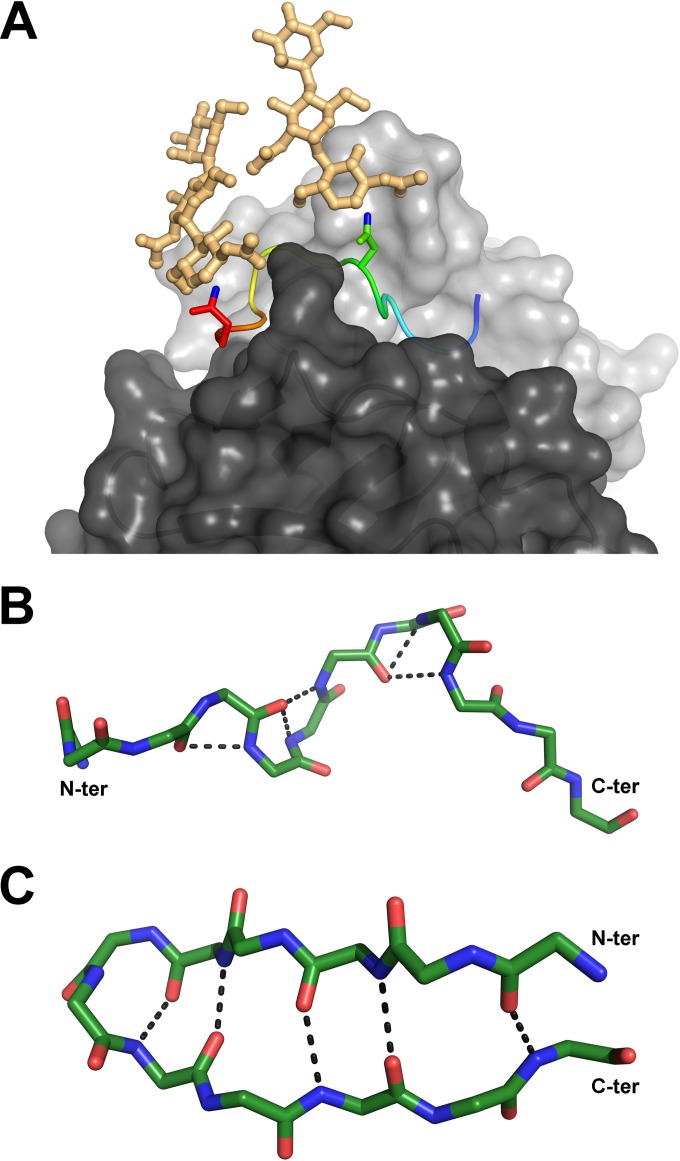
FIG 4.
Relevant conformations of the antigenic site spanning aa 412 to 423. (A) Compatibility of the Fab 3/11-peptide complex structure with the position of N-linked glycans attached to N417 and N423 of the native glycoprotein. Shown is a cartoon representation of the peptide, ramp colored from blue to red through yellow, from the N to the C termini, with the two asparagine side chains shown as sticks and the ND2 atoms, to which the sugar chains are linked, shown in blue. Hypothetical glycan chains containing two N-acetylglucosamine moieties and one mannose moiety (light orange) are modeled to visualize the extended conformation of the native glycoprotein required for 3/11 binding. (B and C) Comparison of both peptide conformations observed for aa 412 to 423. The backbone atoms of the peptides bound to Fab 3/11 (B) and Fab HCV1 (C) (15), as an example of the β-hairpin conformation of aa 412 to 423, are shown as sticks and colored by atom type (green, red, and blue for carbon, oxygen, and nitrogen, respectively) to illustrate the differences in the backbone conformations observed for the two structures. The five hydrogen bonds stabilizing the backbone conformation in both structures are indicated as dotted lines.