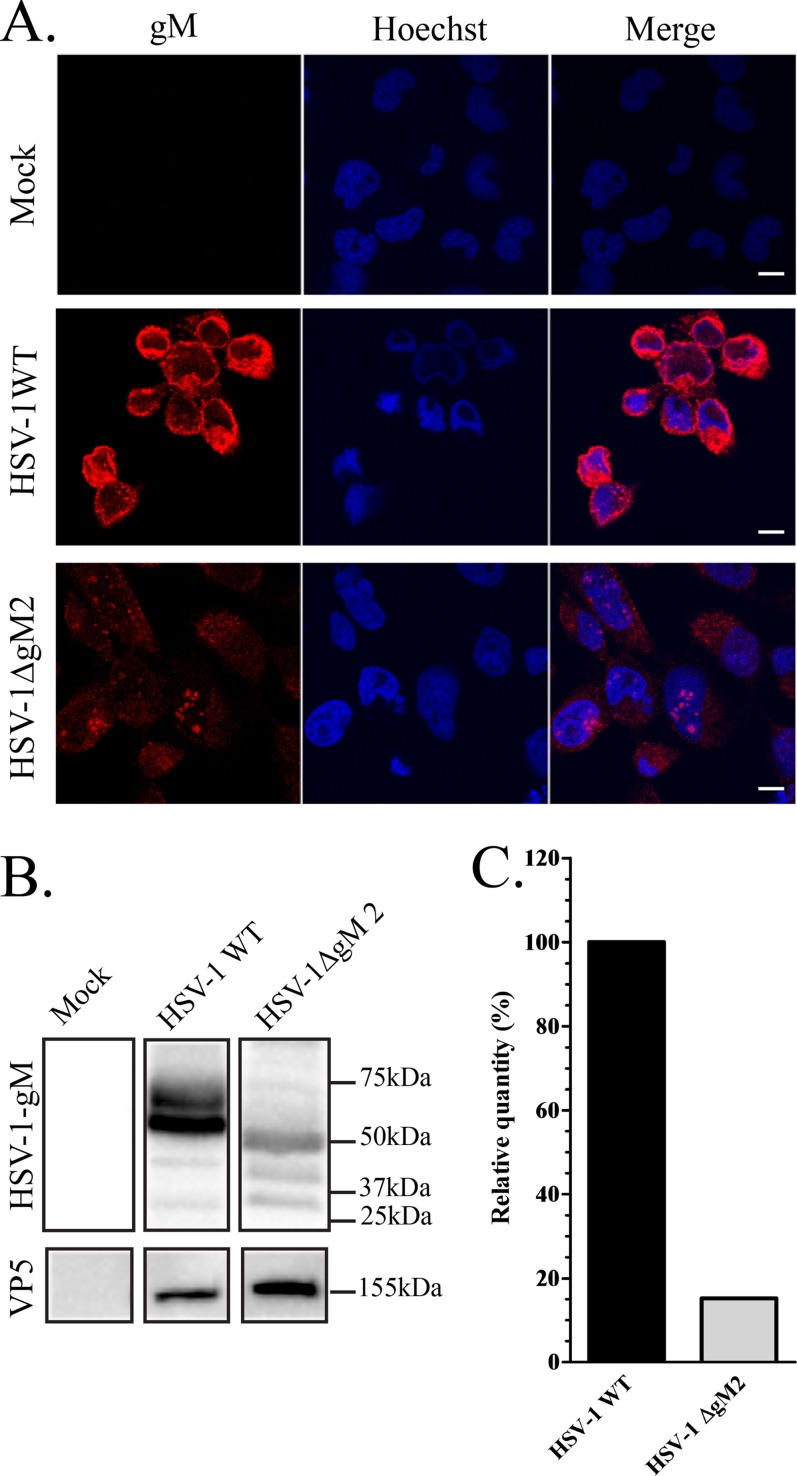
FIG 5.
Expression of gM in wild-type and mutant HSV-1 strains. (A) 143B cells grown on coverslips were infected with the HSV-1ΔgM2 mutant and, as a positive control, wild-type HSV-1. The cells were fixed at 16 hpi and stained with anti-gM (4C10) antibody (red) and Hoechst (blue). (B) HSV-1- or HSV-1ΔgM2 mutant-infected or mock-treated 143B cells were also harvested at 16 hpi, and cell lysates were analyzed by Western blotting. The blots were reacted with anti-gM (PAS980) antibodies or, as a loading control, antibodies against the major capsid protein VP5. Mock-infected cells were used as a negative control. Note that the predicted molecular mass for gM is 52 kDa, while that of the truncated HSV-1ΔgM2 mutant (lacking the first 132 amino acids) is 37.5 kDa. These predictions do not take into consideration the reported post-translational processing of the gM protein, clearly evident in the blots. (C) Quantification of the Western blot expression levels of gM in HSV-1 WT and the HSV-1ΔgM2 mutant. These estimates were normalized against VP5 (ratios of gM to VP5). For easier comparison, the data for wild-type virus was arbitrarily defined as 100%. Scale bar, 10 μm.