Figure 4.
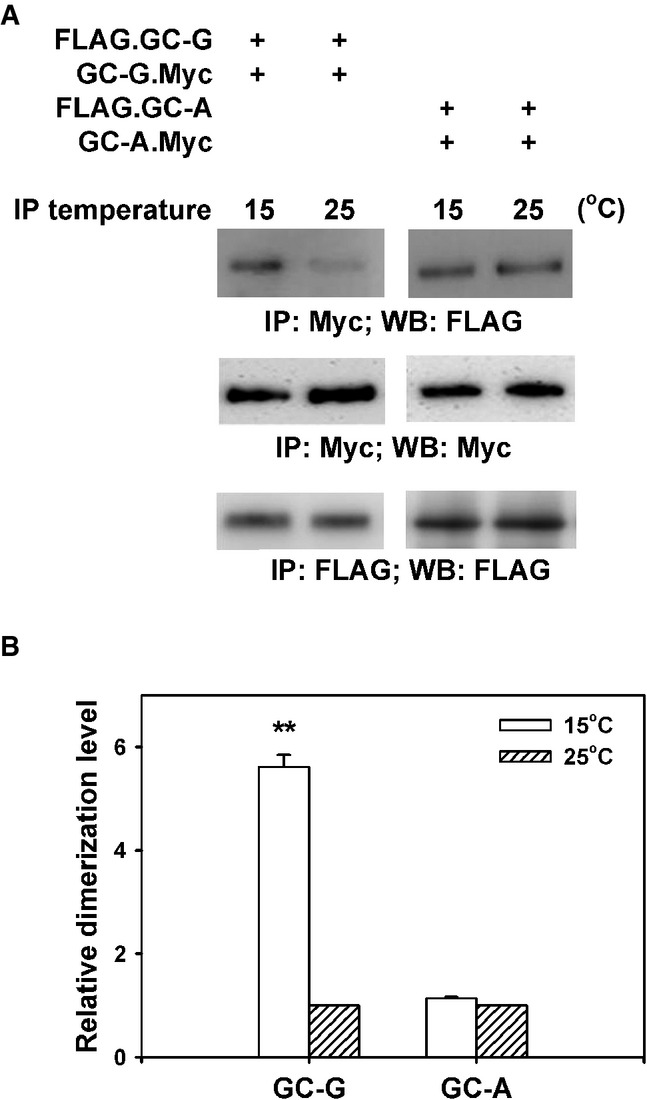
- HEK-293T cells were co-transfected with plasmids encoding two differential epitope-tagged (FLAG or Myc) versions of GC-G or GC-A, respectively. After 2 days, transfected cells were lysed with detergent and lysates were subjected to immunoprecipitation (IP) for 2 h with anti-Myc antibody at two different temperatures (15 or 25°C), followed by Western blotting (WB) using anti-FLAG antibody to determine the amount of dimerized/oligomerized GC proteins (upper panel). These approaches demonstrated that in contrast to GC-A, homomeric protein/protein association of GC-G seems to be enhanced by cool temperatures (upper panel). Cell lysates werealso precipitated and immunoblotted with the anti-Myc antibody, demonstrating that cooling does not interfere with immunoprecipitation of Myc-tagged GC-G via the anti-Myc antibody (middle panel). Likewise, precipitation and immunoblotting with the anti-FLAG antibody yielded bands of similar intensity for 15 and 25°C, respectively (lower panel).
- Relative quantification of homomeric protein/protein association of GC-G and GC-A was performed by densitometric scanning. Each intensity value from the upper panel was subsequently normalized to the total protein levels as determined in the middle and the lower panel of Fig4A (intensity of the immunoreactive bands obtained with the anti-Myc or anti-FLAG antibody, respectively). Relative dimerization level at 15°C was further calculated by normalizing to values obtained at 25°C; the latter were set as 1. Results are means ± SD of three experiments, **P < 0.01 (compared to 25°C).
