Figure 3.
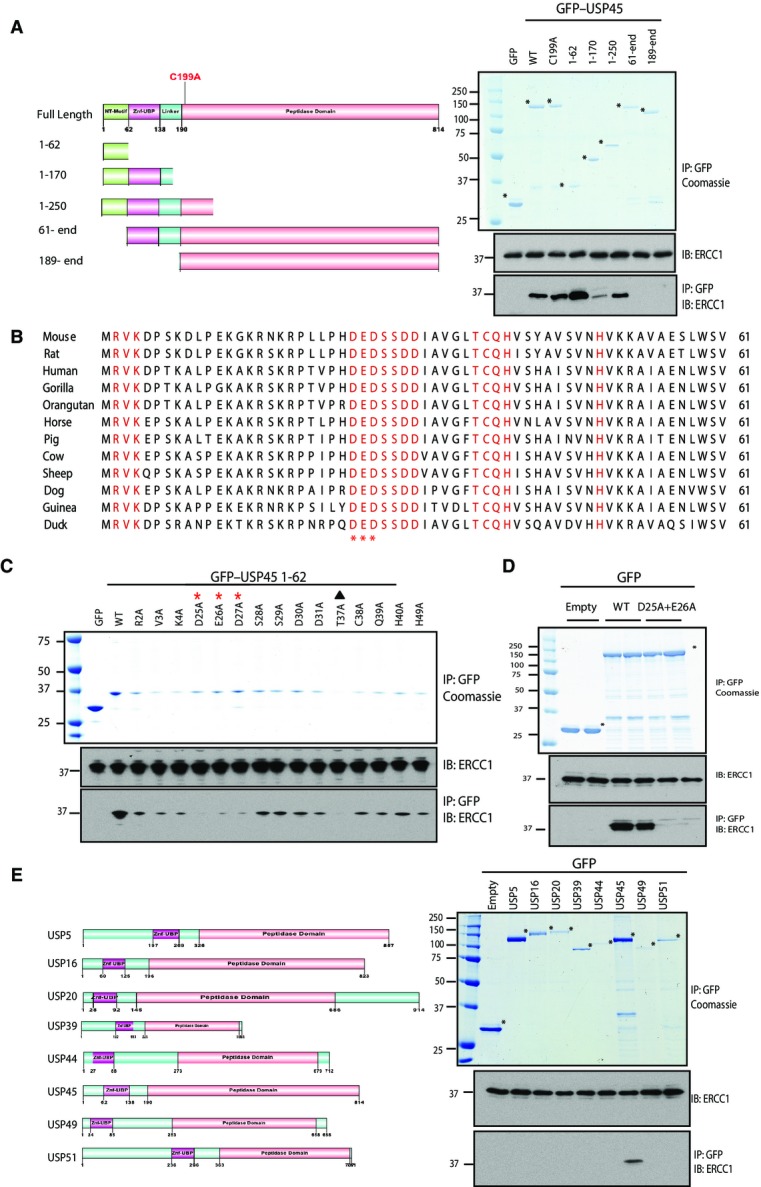
- HEK293 cells were transiently transfected with the indicated GFP fusion constructs encoding full-length or indicated fragments of USP45. Thirty-six hours post-transfection cells were lysed and GFP immunoprecipitated. The immunoprecipitates were resolved on a polyacrylamide gel and stained with Instant Blue or subjected to immunoblotting with the indicated antibodies. GFP empty vector was used as a negative control.
- Sequence alignment of the first 62 residues of USP45 from the indicated species. Conserved residues that were mutated in part (C) are indicated in red. The residues whose mutation in (C) is shown to inhibit binding to ERCC1 are marked with an asterisk. Residue Thr37 (marked with a solid triangle) was found to destabilise expression when mutated to Ala.
- HEK293 cells were transiently transfected with constructs expressing the indicated wild-type or mutant GFP–USP45 [1–62] fragment (C) or GFP full-length USP45 (D) or empty GFP (C and D) as a control and processed as described in (A). LICOR–Odyssey quantitation of results shown in (C) is shown in Supplementary Fig S4.
- As in (C) except HEK293 cells were transfected with the indicated N-terminal Znf-UBP domain-containing DUBs. Similar results were obtained in at least two separate experiments.
