Abstract
Traditional tissue engineering approaches to the restoration of orthopaedic tissues promise to be expensive and not well suited to treating large numbers of patients. Advances in gene transfer technology offer the prospect of developing expedited techniques in which all manipulations can be performed percutaneously or in a single operation. This rests on the ability gene delivery to provoke the sustained synthesis of relevant gene products in situ without further intervention. Regulated gene expression is also possible, but its urgency is reduced by our ignorance of exactly what levels and periods of expression are needed for specific gene products. This review described various strategies by which gene therapy can be used to expedite the repair and regeneration of orthopaedic tissues. Strategies include the direct injection of vectors into sites of injury, the use of genetically modified, allogeneic cell lines and the intra-operative harvest of autologous tissues that are quickly transduced and returned to the body, either intact or following rapid cell isolation. Data obtained from pre-clinical experiments in animal models have provided much encouragement that such approaches may eventually find clinical application in human and veterinary medicine.
Keywords: Gene therapy, Facilitated endogenous repair, Regenerative orthopaedics, bone, cartilage, arthritis
Introduction
Injuries to the skeletal system are common and the damaged tissues may not heal spontaneously. Articular cartilage, meniscus and intra-articular ligaments, for instance, have little intrinsic ability to repair. Long bones, on the other hand, normally heal fractures very efficiently; nevertheless, approximately 5–10% of fractures result in non-unions. Moreover, large segmental osseous defects and cranial defects do not heal in adults. Extra-articular ligaments and tendons usually heal, but the repair tissue is structurally and functionally inferior to undamaged tissue. In all cases, the ability for spontaneous repair declines with age.
A number of diseases of the skeletal system also lead, directly or indirectly, to loss of structural integrity. Osteoarthritis (OA), for instance, produces large lesions in the articular cartilage and tumour resection often removes considerable amounts of tissue.
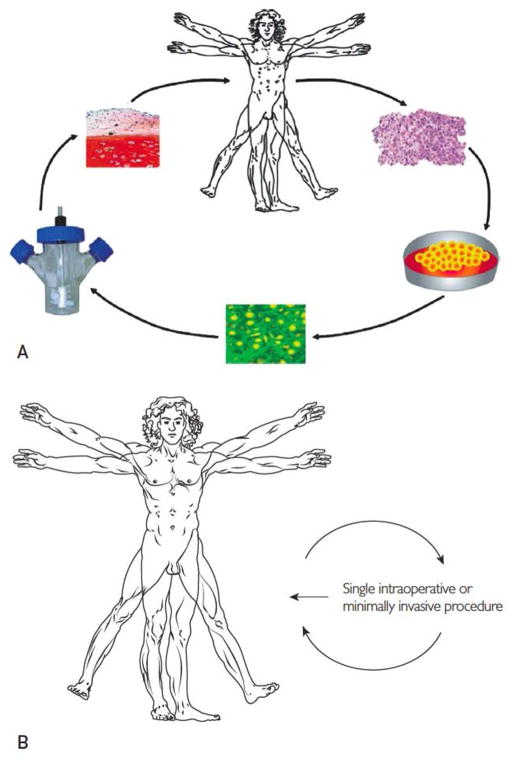
Although certain surgical techniques and engineered devices can mitigate some of these problems, the present trend is to seek biological solutions that promise to restore tissue in a more natural, functional and permanent manner [1]. Tissue engineering has attracted much attention. This has traditionally involved the removal of host cells followed by their in vitro expansion, seeding onto a scaffold, incubation in a bioreactor and implantation (figure 1A). While much progress has been made in this endeavour it is cumbersome and expensive, requiring two invasive procedures, one to harvest cells and the other to implant the engineered tissue. Because the patient population is potentially so large, there is a need for expedited, affordable procedures.
Figure 1. Traditional and expedited approaches to tissue repair and regeneration.
Panel A. In traditional tissue engineering approaches, a first procedure removes tissue biopsies. Autologous cells are isolated, seeded on a matrix and incubated in a bioreactor under conditions where new tissue is formed. The graft is implanted into the patient in a second procedure.
Panel B. Expedited approaches require a single percutaneous, minimally invasive, or intra-operative procedure.
From reference 1, as modified from reference 5, with permission.
It has long been recognised that gene transfer has the potential to enhance the process of tissue engineering [2–4]. Our group is exploring methods that can be implemented in a minimally invasive fashion or during a single operation (figure 1B). The key requirement is that nothing leaves the operating theatre. A favoured strategy attempts to provoke the intrinsic regenerative potential of the tissue in question, an approach that has been termed “facilitated endogenous repair” [5]. Gene transfer is a key component of this approach.
Advantages of gene delivery
Although gene therapy is most often considered in the setting of genetic diseases, it also has a potential role in treating acquired conditions. In this context, it serves as a delivery system for the product of the gene in question. This might be a protein, such as a transcription factor, enzyme or growth factor, or a species of non-coding RNA that regulates the expression of other genes. There are several advantages to delivering a gene rather than its cognate gene product, whether protein or RNA.
Delivery of a gene product usually has brief biological influence, because the product is rapidly cleared. To achieve sustained effects it is necessary to apply repeatedly the molecules in question or implant some sort of controlled delivery device. Much research focuses on the latter, but has so far had limited success in the regenerative orthopaedic arena [6].
Gene transfer offers several advantages for facilitated endogenous repair. The first relates to the limited opportunity for cell manipulation during a single, expedited procedure. Based on conversations with orthopaedic colleagues, it seems that a single operation allows up to 2 hours to complete all necessary procedures. Whatever occurs during this short window needs to have a prolonged downstream effect during the ensuing weeks or months during which new tissue is formed.
Advances in gene transfer technology mean that it is now possible to transduce cells rapidly. Once a gene or cDNA has been transferred to the cell nucleus it will continue to produce the gene product for as long as the cell exists, and the gene remains present and expressed. This offers the possibility of not only sustained expression, but also regulated expression in which synthesis of the encoded transgene product can be regulated temporally and quantitatively by small molecules or other such signals. It is also possible to envision endogenous control of transgene expression by local cues. Urgency to invest in regulated gene expression is reduced by lack of information concerning the expression profiles required of specific gene products. Nearly all studies where gene therapy is used for regenerative orthopaedics have used strong constitutive promoters and, apart from the work of Gazit and colleagues [7, 8], regulated gene expression remains little explored.
Gene transfer also facilitates the delivery of gene products with intracellular sites of action. Important examples include transcription factors and non-coding RNA. Although proteins can be modified to include protein transduction domains and RNA can be delivered by transfection, these methods can be inefficient and, as noted above, need to be repeated in anything beyond an acute condition.
Unlike many of the recombinant proteins used clinically, proteins synthesised following gene delivery are more likely to have undergone authentic post-translational modification. This often leads to greater biological activity and reduces concerns over possible immunogenicity. Moreover, purification, sterilisation and storage of the protein are no longer issues, although these matters still arise for the vectors used for gene delivery, described next.
Gene delivery vectors
Vectors are the vehicles that transfer exogenous DNA (transgenes) into target cells, with transport to the nucleus of the cell where gene expression is initiated. The most powerful vectors are derived from viruses, taking advantage of the innate ability of viruses to deliver their own genomes efficiently to the cells they infect. To create a vector for gene therapy, the viral genome is modified to remove sequences that contribute to virulence and disease thereby creating genetic room for therapeutic sequences. An increasing number of viruses have been modified in this fashion [9]. Those that have advanced to testing in human clinical trials [10] include retrovirus, lentivirus, adenovirus, vaccinia virus, adeno-associated virus (AAV), herpes simplex virus, and measles virus, among others. Gene transfer using a virus is known as transduction.
Because viral vectors are often complicated to manufacture, expensive to produce and attract safety concerns, there is continuing interest in using non-viral vectors [11]. They raise far fewer safety issues than viral vectors, an important consideration in context of regenerative orthopaedics, which addresses non-lethal conditions; they are also much cheaper to produce. Non-viral vectors can be as simple as naked DNA. Transfer efficiency can be increased by combining the DNA with a polymeric carrier or by using physical stimuli such as electroporation or sonication. Although non-viral vectors hold much potential, in our experience they remain inefficient and transgene expression is transient. For this reason we have focussed on viral vectors. Gene transfer using a non-viral vector is known as transfection.
Safety is of paramount concern in the context of regenerative orthopaedics, which places constraints on the selection of viral vectors. The risk of insertional mutagenesis will make regulatory approval very difficult for so-called integrating viruses, which insert their genomes into those of the cells they infect. Retroviruses and lentiviruses (a type of retrovirus) are examples of integrating viruses used for gene therapy. One potential advantage of viral integration is the transmission of the transgene to daughter cells after division of the host cell, thereby aiding long-term transgene expression. This, however, may not be necessary for the repair and regeneration of orthopaedic tissues; indeed, prolonged production of a growth factor, for example, could be detrimental. Lentiviruses have been engineered to become non-integrating while retaining their high transduction efficiency.
AAV has the advantage of causing no known disease. Recombinant AAV transduces both dividing and non-dividing cells, and persists as an episome in the nuclei of transduced cells for extended periods. The disadvantages of this vector include its small DNA carrying capacity and expensive, complicated manufacture.
Vectors derived from baculoviruses, which normally infect insects, are gaining in popularity. They have large DNA genomes, but are easy to manipulate genetically and can be readily produced at high titers. Very high levels of transgene expression can be achieved. Their application to the regeneration of bone and cartilage has been reviewed recently by Lin et al. [12].
Our group has favoured vectors based on adenovirus for regenerative orthopaedics. This virus infects many different types of dividing and non-dividing cells. Expression of transgenes is usually high. First and second generation recombinant adenovirus vectors are straightforward to produce and have been used in over 400 human clinical trials [10]. Under normal circumstances, in vivo transgene expression using early generation adenovirus vectors is high for 1–2 weeks and then declines, with little transgene expression after 3–6 weeks. These kinetics may be ideal for many applications in regenerative orthopaedics.
Gene delivery strategies
Regardless of the vector that is used, genes may be transferred in an in vivo or ex vivo fashion. The traditional tissue engineering approach to repair (figure 1A) lends itself to ex vivo gene transfer, and this has been evaluated experimentally. However, unless there are special circumstances relating, for example, to cost or a need for intracellular location of the gene product, it may be more convenient to expose the cells to recombinant proteins or RNA transfection in the bioreactor than to attempt gene delivery.
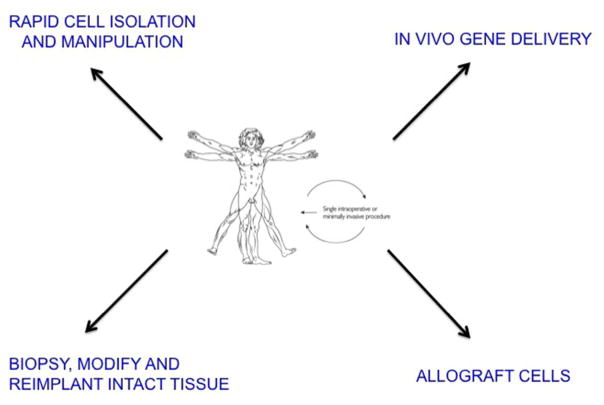
There is increasing interest in foregoing a prolonged bioreactor step and instead implanting expanded autologous cells, such as mesenchymal stem cells (MSCs), on suitable scaffolds without further differentiation of the cells. Tissue formation and maturation then occur in vivo. This strategy also provides the opportunity for ex vivo gene delivery and the two technologies complement each other well in this regard. The drawback, however, is the need to recover and expand each individual’s cells in a Good Manufacturing Practice (GMP) facility, which makes the approach tedious and expensive. One way around this is to use allograft cells from a universal donor (figure 2). As described later in this review, such an approach is being evaluated clinically in the context of cartilage repair. The degree to which an allograft response limits the usefulness of this general strategy remains to be seen.
Figure 2.
Strategies for the facilitated, endogenous repair of tissues
In vivo gene delivery is simpler, but complicates the regulatory pathway because infectious agents are introduced directly into the body. Moreover, many viral vectors are immunogenic and humans often have pre-existing immunity to the most common viruses used for gene transfer. Even if they do not, the initial application will generate immunity and thus complicate repeat dosing, should this be necessary. Another limiting factor is the need for local host cells that can be transduced by the injected vectors. It is likely that gene therapy will be reserved recalcitrant clinical cases, often associated with considerable cell death in and around the injury site. Under these conditions, an in vivo approach may be ineffective. A big advantage of ex vivo delivery is its ability to deliver cells in addition to genes.
Facilitated endogenous repair
Based on the discussion so far, the approach favoured by our group requires a strategy whereby genetically modified cells can be introduced intra-operatively or percutaneously into a defect within a 2-hour window (figure 1B). Figure 2 illustrates different ways in which this might be accomplished.
In vivo delivery
As noted, in vivo gene delivery offers a straightforward approach to tissue regeneration that can be accomplished percutaneously [13] or intraoperatively [14].
This strategy has been applied to the healing of segmental osseous defects in rat [15–18], lapine [17, 19–22], equine [23–25] and ovine [26, 27] models. Studies in the rat explored the direct, intra-lesional injection of recombinant adenovirus carrying cDNA encoding human bone morphogenetic protein-2 (Ad.BMP-2). This showed good efficacy in about half of the animals, but delayed union in the others; the latter had a prominent band of cartilage suggesting a possible pseudarthrosis. This method was ineffective in a sheep tibial defect [27]. Of interest, it had a beneficial effect in sheep treated with high doses of methylprednisolone as part of a protocol to induce osteoporosis [26]. Among the explanations of this phenomenon is the possibility that the glucocorticoid had suppressed the immune response to the virus. In contrast to sheep, good results have been reported for the healing of osteochondral defects in the horse after direct application of Ad.BMP-2 or Ad.BMP-6 [23]. Delaying the introduction of the virus seems advantageous, possibly because it allows time for progenitor cells to enter the defect [15, 23].
Madry and Cucchiarini have explored direct gene delivery in the context of cartilage repair [28]. Using a rabbit model, these investigators have shown it possible to deliver recombinant AAV vectors directly to cells as they enter full thickness cartilagenous defects in rabbit knees. Particularly impressive results were found with fibroblast growth factor-2 (FGF-2) cDNA as the transgene [29]. This strategy could be used in conjunction with microfracture to accelerate healing and enhance the quality of the repair cartilage. AAV is of particular interest in cartilage repair strategies, because it is small enough to penetrate the matrix of cartilage and transduce cells in situ [30, 31]. In this context it has been evaluated in vitro for possible utility in the repair of meniscal lesions [32]. Adenovirus, in contrast, penetrates meniscus to a very limited degree, even after injection into the matrix [33].
The nucleus pulposus of the intervertebral disc supports the in situ transduction of cells by recombinant adenovirus [34] and AAV [35]. Very promising results have been obtained in preventing lapine models of disc degeneration using AAV to deliver cDNAs encoding combinations of BMP-2 and tissue inhibitor of metalloproteinases (TIMP)-1 [35] or BMP-7 and the transcription factor sox 9 [36]. Additional data suggest that the interleukin-1 receptor antagonist (IL-1Ra) cDNA could be useful in preventing disc degeneration [37, 38]. This is interesting in view of the AAV.IL-1Ra vector that is moving towards clinical trials for OA [39].
As reviewed in references [40, 41], in vivo gene delivery to ligament and tendon has been reported for adenovirus, lentivirus, AAV and several non-viral formulations.
Use of allograft, universal donor cells
As noted above, the approach of using transduced allograft cells is in clinical trials. Early research using rabbit models confirmed that genetically modified, allogeneic chondrocytes could be transferred to cartilagenous defects where they continue to express marker genes in the host defect site [42, 43]. Subsequent studies confirmed that use of a cDNA encoding a chondrogenic factor, such as insulin-like growth factor-1 (IGF-1) [44–47], FGF-2 [48] or bone morphogenetic protein-7 (BMP-7) [49, 50] could improve repair of a cartilagenous defect by autologous cells.
These approaches have been taken into human clinical trials by the companies Kolon in Korea and TissueGene in the USA (clinicaltrials.gov identifier NCT01671072). The technology uses lines of articular chondrocytes grown from finger joints recovered surgically from a new-born child with polydactyly. One cohort of cells was transduced with a retrovirus carrying cDNA encoding transforming growth factor-β1 (TGF-β1). Because the virus vector is integrating, the cells are irradiated prior to use to prevent them from dividing. This eliminates the possible development of tumours, should insertional mutagenesis have occurred. To amplify and extend the effects of the irradiated cells, they are mixed with untransduced, unirradiated cells before use.
These cells have been used in two different ways. In patients with OA, cell suspensions are injected directly into the joint [51]. For cartilage injuries, the cells are implanted into defects in a manner analogous to articular chondrocyte implantation. Both applications have completed phase II studies in humans and are about to enter phase III trials.
Allografted cells have also been explored in pre-clinical studies of bone healing. Lieberman’s group pioneered the use of genetically modified, autologous, marrow-derived MSCs for healing large segmental defects [52, 53]. Using a rat model, Tsuchida et al. [54] explored the possibility of employing allografted MSCs. Like Lieberman, they used a recombinant adenovirus vector (Ad.BMP-2) to deliver a human BMP-2 cDNA to marrow-derived MSCs. The data showed that allografted cells were only able to repair the defect when transient immunosuppression with FK506 was imposed.
One way to overcome this impediment to the use of allograft cells is indicated by the recent publication of Sonnet et al. [55] who protected the cells by encapsulation in poly (ethylene glycol) diacrylate. When protected donor cells were transduced with Ad.BMP-2 and implanted as encapsulated microspheres into a rat femoral defect, healing occurred rapidly and efficiently. However, in this configuration, the implant serves only as a local source of BMP-2, with no contribution of donor cells to the regenerate. The relative importance of donor cells as suppliers of trophic factors or as sources of new tissue is much debated and remains unresolved.
Rapid cell isolation and manipulation
MSCs are a popular source of progenitor cells for the repair of orthopaedic tissues. Lieberman’s group have developed an expedited procedure in which populations of cells enriched for MSCs are recovered from bone marrow, transduced with lentivirus carrying BMP-2 cDNA and returned to defects on a collagenous scaffold. Proof of principle has been demonstrated in rat segmental defects [56].
We have developed an analogous approach using fat as the starting material. In a single session, this can be rapidly recovered, digested with collagenase, transduced with Ad.BMP-2, encapsulated in fibrin and returned to segmental defects in rats [57].
Biopsy, modify and reimplant intact tissue
An alternative approach makes use of tissues that can be readily biopsied and genetically modified without the need to isolate cells. If they also have scaffolding properties, they can then be inserted into the defect without further manipulation. Skeletal muscle, fat and marrow are of interest in this regard (figure 3), marrow acquiring scaffolding properties after it clots.
Figure 3. Facilitated, endogenous repair using genetically modified muscle, fat and bone marrow.
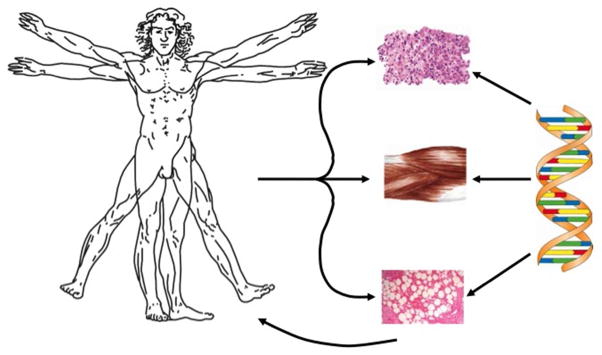
Modified from reference 5, with permission.
Skeletal muscle is of particular interest in the context of bone healing, because of the genetic disease fibrodysplasia ossificans progressiva (FOP) in which skeletal muscle spontaneously turns to bone. FOP is caused by a mutation that increases responsiveness to BMP-2 [58]. This suggests that bone forms intra-muscularly in skeletal muscle in humans in response to a sustained BMP-2 signal. The fact that this occurs in humans is especially important, given the deficiencies of animal models and species differences in response to growth factors such as BMP-2. The osteogenic predeliction of muscle is also demonstrated by the high incidence of heterotropic ossification following blast injuries [59] and certain types of surgery [60].
The value of this has been demonstrated in a rat model, where muscle biopsies were recovered from donor rats, transduced with Ad.BMP-2 and inserted into critical size segmental defects [61]. Reliable and rapid healing was achieved. Fischer rats were used in these experiments because these animals constitute a syngeneic strain allowing tissue transfer between individuals as a surrogate for autologous grafting. Sprague-Dawley rats are not syngeneic and the procedure was considerably less effective in this strain, indicating the sensitivity of the osteogenic response to immune activation as shown by the work of Tsuchida et al. [54], noted earlier. Genetically modified fat biopsies were also effective in many animals, but this was less reliable than when using muscle.
Because healing of segmental osseous defects with genetically modified fat and muscle was found to occur through an endochondral mechanism, we tested whether these grafts could also heal cartilage lesions. Preliminary experiments in rabbits suggested that this was indeed the case [61].
Preliminary experiments have been performed using genetically modified muscle for tendon healing Achilles tendon in rats [41].
The concept of using clotted marrow arose in the context of cartilage repair where microfracture techniques are commonly used to heal cartilage lesions. As a result of microfracture, a clot forms in the lesion; progenitor cells enter from the marrow and differentiate into cells that resemble chondrocytes but produce a fibrocartilagenous repair tissue that eventually fails. We suggested that these cells could form authentic, durable articular cartilage if they were provided with the appropriate morphogens delivered genetically by adenovirus incorporated into the clot. Moreover, placing clotted morrow directly into the lesion promises to accelerate the healing process that would otherwise be delayed by the time taken for cells to migrate into the lesion from the marrow.
To test these possibilities, bone marrow was recovered from rabbits, mixed with adenovirus vectors and allowed to from a clot, known as a “gene plug” [62]. The properties of the gene plug are such that it can be press-fit into the lesion; moreover the shape and dimensions of the vessel in which clotting occurred could be selected to match the requirements of the defect. Preliminary experiments in rabbits confirmed efficient gene transfer to cells within the clot, possibly reflecting the high transduction efficiency of adenovirus when associated with fibrin [63].
This concept was further extended to the healing of partial thickness defects in a sheep model, using TGF-β1 as the transgene [64]. This confirmed the feasibility of the technology in large animals and led to improved healing of the cartilage.
Blood clots incorporating adenovirus have been explored for gene delivery to meniscus [33].
Perspective
In addition to being scientifically sound and clinically effective, advanced technologies in regenerative orthopaedics need to be affordable, expeditious and capable of treating large numbers of individuals. By avoiding the ex vivo cultivation of autologous cells, the methods described in this review eliminate several of the bottlenecks and costs associated with traditional, tissue engineering approaches. Nevertheless, use of gene therapy raises new ones [39, 65]. However, the field of gene therapy is rapidly maturing and the EMA recently approved its first human gene therapy, glybera, for the treatment of lipoprotein lipase deficiency [66]. As more protocols advance to the clinic, the pathway for approval will become clearer and progress will be expedited. The technologies described in this review attempt to minimise regulatory issues and facilitate translation. Genetically modified, allograft chondrocytes are poised to enter phase III clinical trials, additional trials in the gene therapy of arthritis have completed phase I [67–69] and II [70] and two additional protocols for arthritis are moving towards clinical trials [39]. As momentum builds, progress should accelerate and the scope of orthopaedic gene therapy broaden.
Acknowledgments
The author’s work in this area has been funded by the AO Foundation and NIH grant numbers R01AR50243 and AR 052809. Declan Devine, PhD is thanked for advice and discussion.
References
- 1.Evans CH. Advances in regenerative orthopedics. Mayo Clinic proceedings. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clinical orthopaedics and related research. 1999:S410–418. doi: 10.1097/00003086-199910001-00040. [DOI] [PubMed] [Google Scholar]
- 3.Evans CH, et al. The 2003 Nicolas Andry Award. Orthopaedic gene therapy. Clinical orthopaedics and related research. 2004:316–329. doi: 10.1097/01.blo.0000148854.14399.ec. [DOI] [PubMed] [Google Scholar]
- 4.Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. The Journal of bone and joint surgery. American volume. 1995;77:1103–1114. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Evans CH, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue engineering. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 6.Sohier J, et al. Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert opinion on drug delivery. 2008;5:543–566. doi: 10.1517/17425247.5.5.543. [DOI] [PubMed] [Google Scholar]
- 7.Moutsatsos IK, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Molecular therapy: the journal of the American Society of Gene Therapy. 2001;3:449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 8.Kimelman-Bleich N, et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30:4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Vannucci L, et al. Viral vectors: a look back and ahead on gene transfer technology. The new microbiologica. 2013;36:1–22. [PubMed] [Google Scholar]
- 10.Ginn SL, et al. Gene therapy clinical trials worldwide to 2012 - an update. The journal of gene medicine. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, et al. Non-viral gene delivery methods. Current pharmaceutical biotechnology. 2013;14:46–60. [PubMed] [Google Scholar]
- 12.Lin CY, et al. Baculovirus as a gene delivery vector for cartilage and bone tissue engineering. Current gene therapy. 2010;10:242–254. doi: 10.2174/156652310791321242. [DOI] [PubMed] [Google Scholar]
- 13.Lattermann C, et al. Feasibility of percutaneous gene transfer to an atrophic nonunion in a rabbit. Clinical orthopaedics and related research. 2004:237–243. doi: 10.1097/00003086-200408000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Niyibizi C, et al. Potential role for gene therapy in the enhancement of fracture healing. Clinical orthopaedics and related research. 1998:S148–153. doi: 10.1097/00003086-199810001-00016. [DOI] [PubMed] [Google Scholar]
- 15.Betz OB, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene therapy. 2007;14:1039–1044. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 16.Betz VM, et al. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Human gene therapy. 2007;18:907–915. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 17.Southwood LL, et al. Evaluation of Ad-BMP-2 for enhancing fracture healing in an infected defect fracture rabbit model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:66–72. doi: 10.1016/S0736-0266(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 18.Betz OB, et al. Direct percutaneous gene delivery to enhance healing of segmental bone defects. The Journal of bone and joint surgery. American volume. 2006;88:355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 19.Baltzer AW, et al. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 20.Baltzer AW, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene therapy. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 21.Baltzer AW, et al. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clinical orthopaedics and related research. 2000:S120–125. doi: 10.1097/00003086-200010001-00016. [DOI] [PubMed] [Google Scholar]
- 22.Bertone AL, et al. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:1261–1270. doi: 10.1016/j.orthres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Menendez MI, et al. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2011;19:1066–1075. doi: 10.1016/j.joca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara A, et al. Comparative efficacy of dermal fibroblast-mediated and direct adenoviral bone morphogenetic protein-2 gene therapy for bone regeneration in an equine rib model. Gene therapy. 2010;17:733–744. doi: 10.1038/gt.2010.13. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara A, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2008;26:764–771. doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 26.Egermann M, et al. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Human gene therapy. 2006;17:507–517. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 27.Egermann M, et al. Effect of BMP-2 gene transfer on bone healing in sheep. Gene therapy. 2006;13:1290–1299. doi: 10.1038/sj.gt.3302785. [DOI] [PubMed] [Google Scholar]
- 28.Cucchiarini M, Madry H. Gene therapy for cartilage defects. The journal of gene medicine. 2005;7:1495–1509. doi: 10.1002/jgm.824. [DOI] [PubMed] [Google Scholar]
- 29.Cucchiarini M, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Molecular therapy: the journal of the American Society of Gene Therapy. 2005;12:229–238. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Madry H, et al. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Human gene therapy. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- 31.Watson RS, et al. scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene therapy. 2013;20:670–677. doi: 10.1038/gt.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madry H, et al. Menisci are efficiently transduced by recombinant adeno-associated virus vectors in vitro and in vivo. The American journal of sports medicine. 2004;32:1860–1865. doi: 10.1177/0363546504265189. [DOI] [PubMed] [Google Scholar]
- 33.Goto H, et al. Transfer of lacZ marker gene to the meniscus. The Journal of bone and joint surgery. American volume. 1999;81:918–925. doi: 10.2106/00004623-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Nishida K, et al. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. discussion 2443. [DOI] [PubMed] [Google Scholar]
- 35.Leckie SK, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. The spine journal: official journal of the North American Spine Society. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren S, et al. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated SOX9 and osteogenic protein-1 double genes in vivo. International journal of molecular medicine. 2013;32:1063–1068. doi: 10.3892/ijmm.2013.1497. [DOI] [PubMed] [Google Scholar]
- 37.Le Maitre CL, et al. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis research & therapy. 2007;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans C. Potential biologic therapies for the intervertebral disc. The Journal of bone and joint surgery. American volume. 2006;88(Suppl 2):95–98. doi: 10.2106/JBJS.E.01328. [DOI] [PubMed] [Google Scholar]
- 39.Evans CH, et al. Arthritis gene therapy and its tortuous path into the clinic. Translational research: the journal of laboratory and clinical medicine. 2013;161:205–216. doi: 10.1016/j.trsl.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildebrand KA, et al. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene therapy. 2004;11:368–378. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- 41.Docheva D, et al. Biologics for tendon repair. Advanced drug delivery reviews. 2014 doi: 10.1016/j.addr.2014.11.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang R, et al. Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 1997;5:139–143. doi: 10.1016/s1063-4584(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 43.Baragi VM, et al. Transplantation of adenovirally transduced allogeneic chondrocytes into articular cartilage defects in vivo. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 1997;5:275–282. doi: 10.1016/s1063-4584(97)80023-4. [DOI] [PubMed] [Google Scholar]
- 44.Brower-Toland BD, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Human gene therapy. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 45.Goodrich LR, et al. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. The Journal of bone and joint surgery. British volume. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 46.Nixon AJ, et al. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clinical orthopaedics and related research. 2000:S201–213. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 47.Madry H, et al. Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model. European cells & materials. 2013;25:229–247. doi: 10.22203/ecm.v025a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orth P, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2011;19:2119–2130. doi: 10.1007/s00167-011-1448-6. [DOI] [PubMed] [Google Scholar]
- 49.Che JH, et al. Application of tissue-engineered cartilage with BMP-7 gene to repair knee joint cartilage injury in rabbits. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2010;18:496–503. doi: 10.1007/s00167-009-0962-2. [DOI] [PubMed] [Google Scholar]
- 50.Hidaka C, et al. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2003;21:573–583. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 51.Ha CW, et al. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14:247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lieberman JR, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. The Journal of bone and joint surgery. American volume. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Lieberman JR, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchida H, et al. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2003;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 55.Sonnet C, et al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31:1597–1604. doi: 10.1002/jor.22407. [DOI] [PubMed] [Google Scholar]
- 56.Virk MS, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schinhan M, et al. Fibrin-encapsulated, genetically modified adipose-derived stem cells for use in tissue repair (abstract) Journal of tissue engineering and regenerative medicine. 2012;6:265. [Google Scholar]
- 58.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature genetics. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 59.Forsberg JA, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. The Journal of bone and joint surgery. American volume. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 60.Zeckey C, et al. Heterotopic ossifications following implant surgery--epidemiology, therapeutical approaches and current concepts. Seminars in immunopathology. 2011;33:273–286. doi: 10.1007/s00281-011-0240-5. [DOI] [PubMed] [Google Scholar]
- 61.Evans CH, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. European cells & materials. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascher A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene therapy. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 63.Neumann AJ, et al. Enhanced adenovirus transduction of hMSCs using 3D hydrogel cell carriers. Molecular biotechnology. 2013;53:207–216. doi: 10.1007/s12033-012-9522-y. [DOI] [PubMed] [Google Scholar]
- 64.Ivkovic A, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene therapy. 2010;17:779–789. doi: 10.1038/gt.2010.16. [DOI] [PubMed] [Google Scholar]
- 65.Evans CH, et al. Orthopedic gene therapy--lost in translation? Journal of cellular physiology. 2012;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO molecular medicine. 2013;5:1–3. doi: 10.1002/emmm.201202291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wehling P, et al. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Human gene therapy. 2009;20:97–101. doi: 10.1089/hum.2008.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans CH, et al. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8698–8703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mease PJ, et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Annals of the rheumatic diseases. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- 70.Mease PJ, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. The Journal of rheumatology. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]