Figure 1.
RTEL1 Directly Interacts with TRF2 Predominantly in S Phase
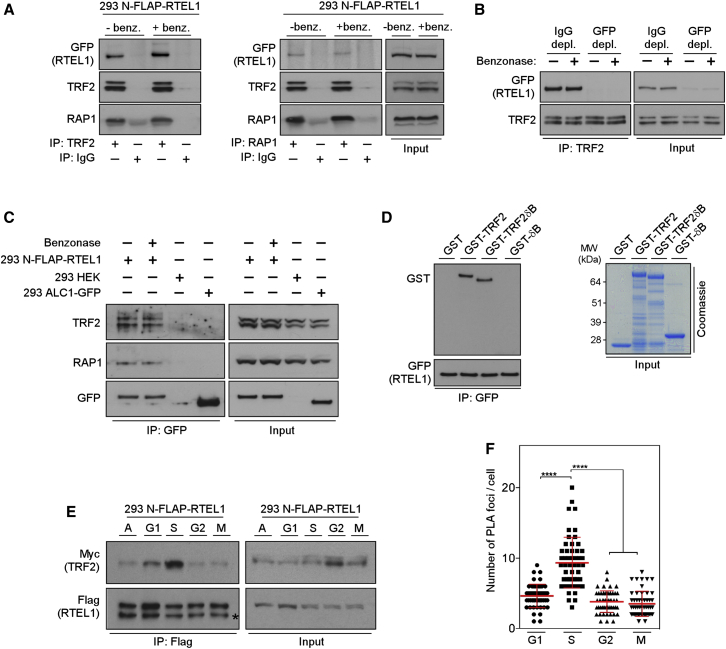
(A) Whole-cell extracts of control (−benz.) and benzonase-treated (+benz.) 293 FLAP-tagged RTEL1 cells were immunoprecipitated using anti-TRF2 or anti-Rap1 antibodies. Protein complexes were analyzed by immunoblotting for GFP, TRF2, and Rap1.
(B) Extracts shown in (A) were immunodepleted with anti-GFP antibody (GFP depl.) or with control IgG (IgG depl.). The GFP- and control-depleted extracts were subjected to immunoprecipitation by anti-TRF2 antibody and analyzed by western blotting as indicated.
(C) Whole-cell extracts of 293 FLAP-tagged RTEL1 cells shown in (A), HEK293 cells, and HEK293 cells transfected with expression vector for ALC1-GFP were immunoprecipitated using GFP-TRAP beads. Immunocomplexes were analyzed by western blotting with indicated antibodies.
(D) Whole-cell extracts from 293 FLAP-tagged RTEL1 cells were incubated with GST control, GST-TRF2, GST-TRF2ΔB, or GST-ΔB. The bound complexes were immunoprecipitated with anti-GFP antibody analyzed by western blotting against GST and GFP.
(E) 293 N-FLAP-RTEL1 cells stably expressing Myc-tagged TRF2 were either cultured asynchronously (A) or released from double-thymidine block (S, G2, and M phase) or from thymidine plus nocodazole block (G1 phase). Cell extracts were analyzed for input (right panels) or were immunoprecipitated with antibodies against Flag (left panel). The immunoblots were probed with anti-Flag and anti-Myc antibodies. Asterisk indicates the nonspecific cross-reactivity detected with Flag antibody.
(F) RTEL1v5 MEFs stably expressing Myc-tagged TRF2 were cultured as indicated in (E). The graph depicts quantification of the interaction spots per cell between RTEL1 and TRF2 as determined by in situ PLA assay. Data are representative of two independent experiments as mean ± SD (∗∗∗∗p < 0.0001, one-way ANOVA). See also Figures S1 and S2.