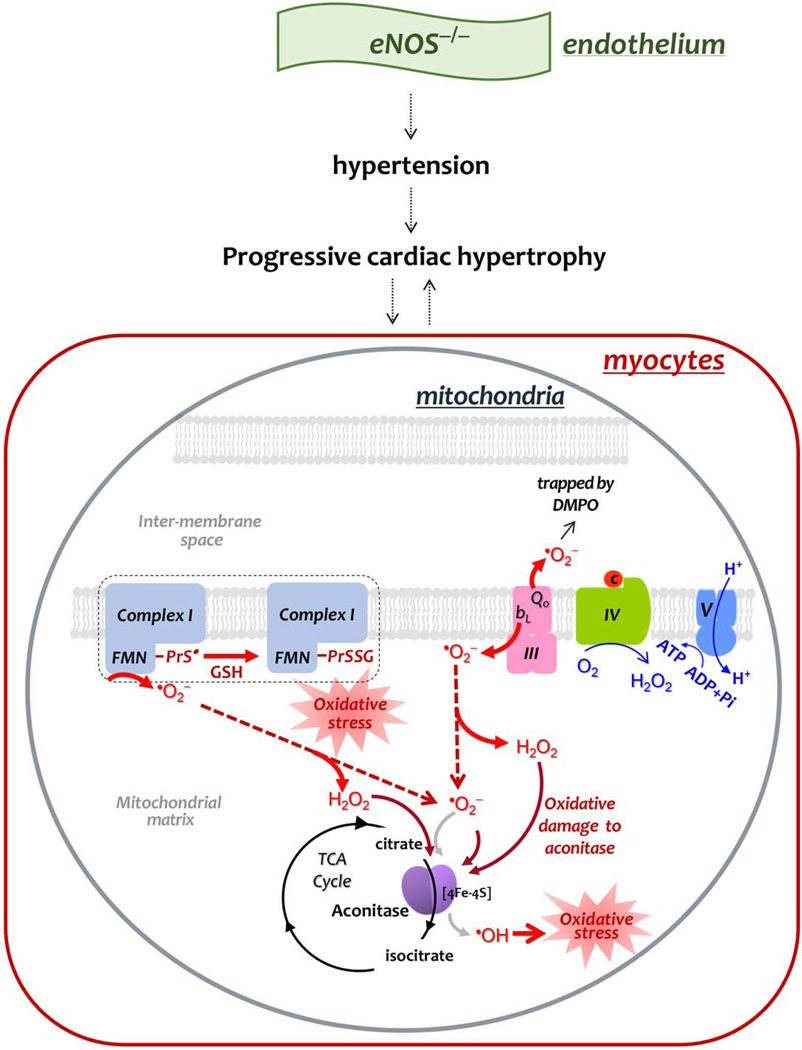
Fig. 9. Diagram showing the effect of eNOS knockout on the mitochondrial function of the mouse heart, and mechanism-based S-glutathionylation of Complex I.
In comparison to the basal conditions, genetic deletion of eNOS impairs EDRF-mediated vasodilation, leading to the phenotype of hypertension, and inducing progressive hypertrophy. The phenotype of progressive cardiac remodeling is likely mediated by elevation of mitochondrial oxidative stress in myocytes or vice versa. Increased oxidative stress decreases the coupling of oxygen consumption with OXPHOS for ATP synthesis (indicated by fine blue arrows in Complex IV and Complex V), which would further increase electron leakage for •O2− production (indicated by thick red arrows). Increased •O2− production by Complexes I and III damages the 4Fe-4S cluster of aconitase in the TCA cycle (thick brick arrows and black cycle), and increases pro-oxidant activity of aconitase to generate •OH (coarse gray arrow), augmenting oxidative stress. Enhanced •O2− production also facilitates Complex I-derived protein thiyl radical formation (PrS•), which mediates enhanced S-glutathionylation (PrSSG) of Complex I (denoted by dashed closed bracket).