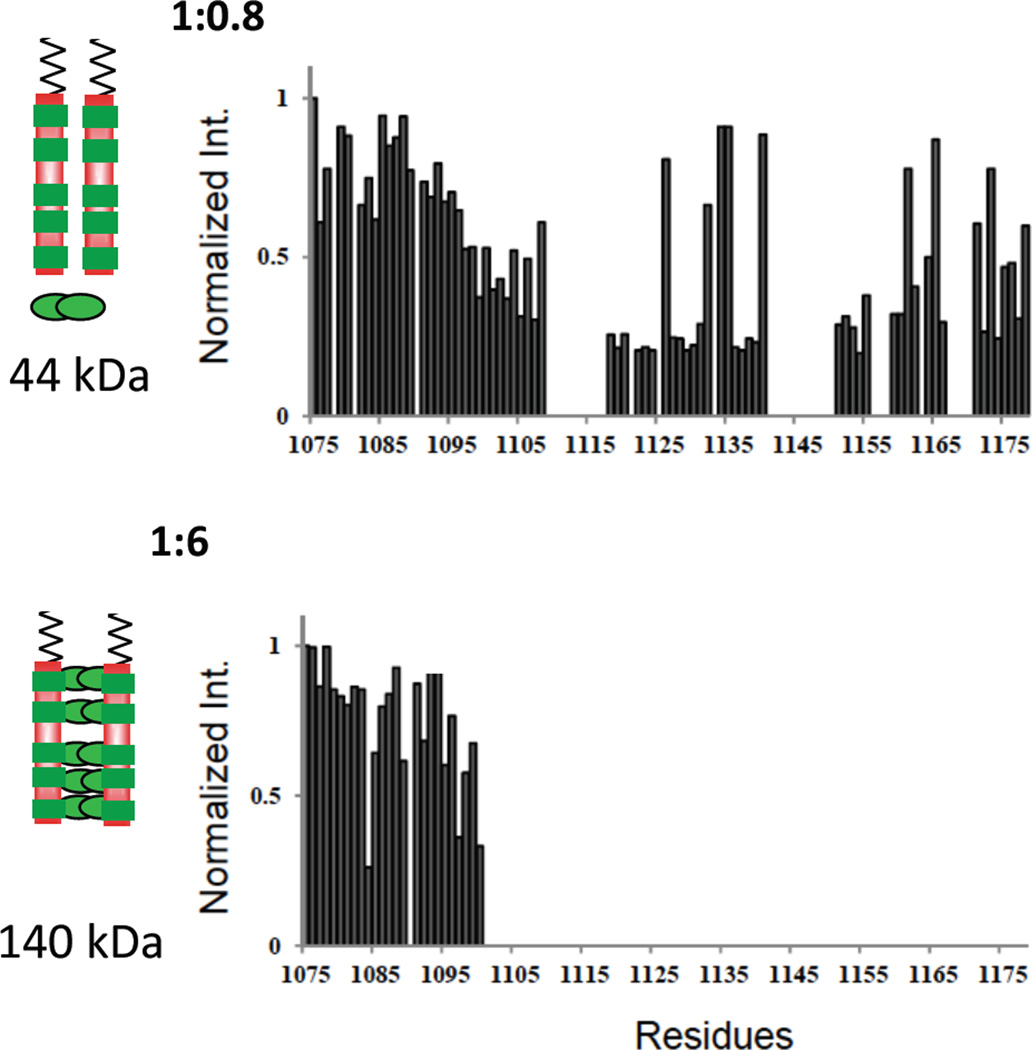
Figure 5.
Residue-specific NMR titration of Nup159 NH peak intensity with added Dyn2. Relative NH peak intensity versus residue numbers of non-proline Nup159 NH peaks in NMR spectra of Dyn2-bound Nup159 in the sequence 1075–1178. Shown are Nup:Dyn2 molar ratios of 1:0.8 (top) and 1:6 (bottom). Peak intensities are relative to the intensity of the same peak in apo Nup159, which is taken as one. This figure is adapted from [9] which also reports results at a 1:2 ratio. The models on the left show two chains of Nup159 (red) with five binding sites for Dyn2 (green). In the top model, a single Dyn2 dimer, indicated at the bottom, is added to two molar equivalents of Nup monomer, and its effect is manifested throughout the Nup159 chain, as indicated regions of attenuated intensity all along the Nup chain. In the bottom model, Dyn2 dimers saturate the five Dyn2 binding sites. The observed loss of peak intensity arises from peak broadening due to exchange, on the NMR time scale, of an NH between different local environments (e.g., in bound and unbound conformations), and/or from an increased population within the ensemble of Nup conformations having significantly greater rotational correlation time [20]. In the latter case, the NH being measured might, for example, fractionally exist in several-to-many conformations such that the same NH when in disordered and locally flexible conformations gives a sharp ‘random coil’ signal, and when in more collapsed and/or aggregated conformations with a significantly longer tumbling time and faster relaxation time gives a broadened signal.