Table 2.
Activity and cytotoxicity of T3SS inhibitors with varying substituents on the phenoxide ring.
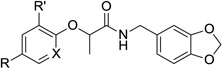 |
||||||
|---|---|---|---|---|---|---|
| Compound # |
X | R | R’ | secretion IC50 (µM) |
translocation IC50 (µM) |
translocation CC50 (µM) |
| 1 | CH | −Cl | −Cl | 7.8 ± 2.0 | 11 ± 2 | >100 |
| 12a | CH | −H | −Cl | 27.0 +/−4.2 | >100 | >100 |
| 12b | CH | −Cl | −H | 70.1 +/−0.7 | >65 | 65.0 +/−4.2 |
| 12c | CH | −F | −Cl | 34 ± 1 | >100 | >100 |
| 12d | CH | −Cl | −F | >100 | n.d. | n.d. |
| 12e | CH | −F | −F | >100 | n.d. | n.d. |
| 12f | CH | −Me | −Cl | 34.0 +/−4.4 | >100 | >100 |
| 12g | CH | −CN | −Cl | >100 | n.d. | n.d. |
| 12h | CH | −OMe | −Cl | >100 | n.d. | n.d. |
| 16 | N | −Cl | −Cl | 5.6 ± 0.2 | 22 ± 10 | >100 |
n.d.: value not determined
