Table 4.
Activity and cytotoxicity of T3SS inhibitors with varying substituents at the benzylic position.
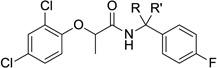 |
|||||
|---|---|---|---|---|---|
| Compound # |
R | R’ | secretion IC50 (µM) |
translocation IC50 (µM) |
translocation CC50 (µM) |
| 2 | −H | −H | 9.8 ± 2.9 | 6.7 ± 1.0 | 59 ± 16 |
| 27a | −Me | −H | 6.0 ± 1.2 | 23 ± 1 | >100 |
| 27b* | −Me (S) | −H | 4.2 ± 0.2 | >32 | 32 ± 1 |
| 27c* | −Me (R) | −H | 2.6 ± 0.9 | >97 | 97 ± 9 |
| 27d | −Me | −Me | 3.3 ± 0.6 | >100 | >100 |
| 27e | −CH2CH2- | 5.4 ± 0.2 | >28 | 28 ± 14 | |
| 27f | −Et | −H | 4.4 ± 0.6 | 25 ± 1 | >100 |
| 27g | −nPr | −H | 3.9 ± 0.7 | >100 | >100 |
| 27h | −iPr | −H | 3.5 ± 1.9 | >100 | >100 |
| 27i | −cPr | −H | 3.0 ± 1.8 | 8.3 ± 2.3 | >100 |
| 27j | −nBu | −H | 4.5 ± 0.4 | >100 | >100 |
| 27k | −cBu | −H | 2.7 ± 0.7 | >100 | >100 |
| 27l | −CH2OH | −H | 6.0 ± 1.5 | >18 | 18 ± 2 |
| 27m | −CN | −H | 5.8 ± 1.0 | 12 ± 2 | >100 |
| 27n | −COOMe | −H | 8.1 ± 2.0 | >100 | >100 |
| 27o | −COOH | −H | >100 | n.d. | n.d. |
n.d.: value not determined
Compounds synthesized using (R)-acid
