Abstract
Background
This study examined the feasibility of a legacy-making intervention in children with cancer and the preliminary effects on outcomes related to quality of life.
Procedure
Children (N = 28) ages 7–17 years completed a baseline QOL questionnaire (PedsQL) at T1. After baseline, the intervention group (n = 15) completed a randomized intervention that guided children to answer questions about legacy-making and create a digital story about themselves. A final copy of the digital story was provided to the families. A control group (n = 13) received customary care. Children repeated the questionnaire at T2. Parents (N = 22) of children who completed the intervention completed follow-up survey questions regarding intervention effects.
Results
Feasibility was strong (78% participation; 1 attrition). While differences between the groups in physical, emotional, social, or school functioning change was not statistically significant, the intervention group showed slightly better emotional and school functioning compared to controls. Parents reported that their child’s digital story provided emotional comfort to them (n = 11, 46%), facilitated communication between parents and children (n = 9, 38%), and was a coping strategy for them (n = 4, 17%). Parents reported that the intervention helped children express their feelings (n = 19, 79%), cope (n = 6, 27%), and feel better emotionally (n = 5, 23%).
Conclusions
Our intervention is feasible for children with cancer, is developmentally appropriate for children 7 to 17 years of age, and demonstrates promise to improve quality of life outcomes for children with cancer and their parents.
Keywords: digital storytelling, legacy-making, children, cancer, pediatric oncology, parent caregivers, quality of life
INTRODUCTION
Despite advances in pediatric oncology research and medical treatment, cancer remains the leading cause of non-accidental childhood death [1]. In the United States, more than 500,000 children have life-threatening conditions, and more than 363,000 children are living with cancer [2]. The incidence of pediatric cancer is increasing, with approximately 15,780 new cases annually among children ages 0–19 [1]. Cancer is the leading cause of death by disease in children up to 15 years old, and approximately 1,350 children (ages 0–14) with cancer are projected to die in 2014.
Children living with and dying from advanced cancer experience significant physical, psychological, social, and spiritual suffering [3]. Helping children and families cope effectively across the cancer continuum is challenging and requires innovative and individualized family-centered care. At the end of life, children with cancer often want to have the opportunity to do something good for others or to leave a gift for their loved ones [4]. Bereaved parents and siblings have reported that many children with advanced cancer did things to be remembered, including making crafts for others, willing away belongings, writing letters to loved ones, and giving special gifts [5]. Legacy-making seemed to be a source of inspiration for both children with cancer and their bereaved families. As a result, bereaved family members perceived that legacy-making activities were important and beneficial for children with cancer and their families.
Legacy-making has been explored in adult and pediatric populations [5–9]. Legacy-making in adults has been shown to increase patients’ sense of dignity, purpose, meaning, and will to live, while decreasing suffering and depressive symptoms [7]. Staff of children’s hospitals have reported that legacy-making activities helped ill children cope and communicate and helped their family members cope, communicate, and continue bonds in the case of the children’s deaths [9]. Many children’s hospitals offer legacy-making activities to pediatric oncology patients or their family members, but these activities are not empirically based. Legacy-making interventions have been empirically tested in adults [7] but not pediatrics. Legacy-making has improved quality of life (QOL) outcomes for individuals with life-threatening illnesses and their families [5, 7, 8], but work is needed to examine legacy-making outcomes for pediatric populations. The purpose of this study was to examine the feasibility of a legacy-making intervention in children (ages 7–17 years) with cancer and the preliminary intervention effects on the outcomes related to QOL in the participants.
METHODS
Conceptual Framework
An empirically based dignity model of palliative care provided the framework for Chochinov et al.’s (2005) work on legacy-making, but this framework has not been tested with children. Given that current conceptual approaches do not specifically address legacy-making in children, the conceptual framework in Figure 1 was developed based on synthesis of current theory and evidence to guide our study. Legacy-making has potential direct effects on child perception of parent caregiver benefit. Chochinov and colleagues (2005) found that for dying adult patients, the beneficial effects of protecting the well-being of their loved ones they will leave behind seemed to extend to the end of life. Foster et al. (2009) found that bereaved family members perceived that their children living with cancer were inspired to create legacies via affecting the lives of others. Legacy-making also has potential direct effects on parent caregiver benefit. Previous work has shown that cognitive behavioral interventions for patients may not only benefit patients but also caregivers (Given et al., 2006). Chochinov et al.’s (2005) work suggested that legacy-making interventions for dying adults resulted in caregiver benefit. Legacy-making in children may also benefit caregivers. Foster et al.’s (2009) study suggested that legacy-making of children with cancer benefitted their bereaved families with inspiration. Legacy-making may directly effect children’s QOL, but be mediated by direct or perceived benefits of legacy-making for parent caregivers. Although the study was not designed to test a conceptual framework, the model provided a basis for examining the potential relationships among legacy-making, parent caregiver benefit, child perception of parent caregiver benefit, and child suffering.
Figure 1.
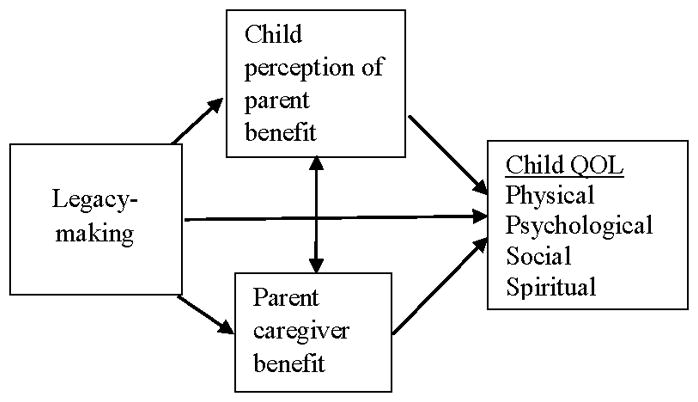
Conceptual framework
Procedure and Measures
Recruitment took place at Monroe Carell Jr. Children’s Hospital at Vanderbilt. The PI received names of potential participants from doctors and nurses in the outpatient pediatric hematology/oncology clinic and from lists of relapsed cancer patients within the electronic medical record system. Eligible participants were (a) children 7 to 17 years of age, (b) diagnosed with any cancer, (c) able to speak and understand English with a parent caregiver able to speak and understand English, and (d) without cognitive impairment and with a parent caregiver without cognitive impairment (determined by chart audits and/or discussions with clinical team). While the intervention was ultimately intended for children considered to have a poor prognosis (e.g., relapsed or refractory disease), children at any point in the illness trajectory were eligible to participate in this study to first examine feasibility among a less vulnerable population.
Parents of children with cancer who meet the inclusion criteria were identified and approached about possible study participation by the primary physician or nurse practitioner during an inpatient hospital stay or clinic visit. If the provider was not available to first approach the family, the principal investigator (PI) or research assistant (RA) approached them after approval from the physician or nurse practitioner. The PI/RA used a script with the following words to describe the study:
… Ultimately, we are trying to develop an activity to decrease suffering of children with cancer and their families in the future. The purpose of this project is (1) develop an activity for children with cancer based on their reported hopes and desires, (2) determine if children are willing and able to complete such an activity….
The PI/RA determined the participating primary parent caregiver by gathering objective information (e.g., number of hours spent per week with the child) during the initial recruitment process. The PI/RA obtained verbal and written parental informed consent and child assent. Permission for a review of the child’s medical record was part of the consent process. A research nurse conducted chart reviews to document patient demographic characteristics (child gender, age, race), cancer disease characteristics (diagnosis, date of diagnosis, recurrence or progression post-treatment, secondary diagnosis, date of secondary diagnosis), treatment characteristics (surgery, chemotherapy, radiotherapy, bone marrow transplant, Phase I studies), and end-of-life care (terminal documentation, hospice care, Do Not Resuscitate (DNR) order in place).
Child participants were randomly assigned to either an intervention or control group by using a computer-generated randomization approach with a permuted block scheme. The randomized intervention was administered to children in the intervention group (n = 15) shortly after baseline measures were completed. A control group (n = 13) received customary care and repeated questionnaires and interviews at T2 (recruited 1 week after T1). Questionnaires were separately administered to the child and parent by the PI/RA in a room selected for privacy. To our knowledge, participants in the control group were not taking part in other psychosocial interventions.
Intervention
Development
A legacy-making intervention via digital storytelling was developed based on suggestions from children (n = 8) with cancer and their parent caregivers [6]. Detailed information regarding development of the intervention can be found in a previous publication [6]. In brief, children with advanced cancer completed individual interviews about what they would like their family and friends to remember most about them and writing words or making something special to give to someone they care about. Children reported that they wanted others to know or remember (a) their personal characteristics (e.g., name, gender, appearance, personal traits), (b) the things that they like to do (e.g., hobbies, interests), and (c) their connectedness with others (e.g., telling family members how much they are loved). Thus, we created guiding interview questions to help children speak about these legacy-related topics (e.g., What is your favorite color? What is your favorite activity or hobby? What special messages would you like to give to other people?). The children (n = 8, 100%) supported the idea of writing words or making something to give to somebody and suggested specific activities involving crafts, computers, music, and games. They preferred to have the choice of working alone or with others. Thus, we selected a digital storytelling intervention format as this choice subsumed most of the offered suggestions and video-recorded scheduled child interviews. Video recordings incorporated the child’s favorite activities, crafts, locations, family members, or pets. A videographer edited the video recordings and incorporated photographs and music selected by the child to create a digital story for the child and his or her family. The final product could be viewed and distributed via the computer and included music and photographs, video, and audio of children’s preferred crafts or games. Digital storytelling allowed intervention participation to be tailored to the unique aspects of each child and family while maintaining consistent intervention fidelity.
Procedure
The PI scheduled the intervention with children in the intervention group at the patient’s earliest convenience after baseline (T1) measures were completed by the child and parent. At T1, participants were given the list of interview questions for the intervention so that they had the opportunity to prepare and think about the questions in advance, as well as what pictures and music they wanted to include. The PI scheduled home assessments (or another private location if requested by the family) for the intervention which averaged 1 hour. The intervention consisted of a videographer video-taping child responses to the guiding interview questions. The PI or trained research nurses conducted the interviews which were part of the intervention. The PI completed the first several interviews, and then used the raw video recordings to train the research nurses on how to do the interviews. Interviewers instructed children that they could skip any questions that they did not want to answer. The child’s family members were allowed to be in the room during the interview or be a part of the video according to the child participant’s preference. Questions included asking children their preferences for music and photographs they wanted to include in their digital story. Intervention integrity was enhanced by (1) the PI delivering the intervention or watching 100% of unedited videos from intervention delivery by the research nurses, and (2) the PI and research nurses following an intervention delivery guide.
The videographer used the video, the child’s song selection, and approximately 12 photographs to create a digital story for each child, taking 6 hours of editing time per story. Although extremely rare, editing included deleting recorded content that might be hurtful to others, which has been referenced as mitigating harm in the dignity therapy literature [7]. Within 1 to 2 weeks of the intervention, the PI provided the family a draft of the child’s newly created digital story by either (1) offering them an UNLISTED YouTube web link (only individuals who have the URL are able to view the video) sent via email, and/or (2) mailing a DVD. The PI followed up with each family via phone or email to gather feedback for any suggested edits to the completed digital story. The videographer made feasible edits. Only 1 family requested further edits to add an “in memory” page for a deceased family member and more family pictures. Then, the families were provided a final copy of the digital story via an emailed UNLISTED YouTube file and mailed DVD. Final copies of digital stories averaged 7 minutes 40 seconds long. Informing child participants that their digital stories would be mailed or emailed to their parent was part of the assent process. The informed assent and consent also included a check box where participants chose to allow or not allow sharing of their video for research purposes or examples to show other children or families. All children and parents in this study agreed to sharing of their video. The control group received customary standard of care and was offered the same intervention within 1 to 2 weeks after T2 measures were completed.
Instruments
PedsQL v. 4.0, Acute Version
The PedsQL is a 23-item generic health-related QOL questionnaire for child self-report [10], and is used to measure child QOL. We used the Child Self Report for ages 5 to 7, 8 to 12, and 13 to 17 years. Children ages 5 to 7 were asked to rate how much each item had been a problem over the past 7 days on a 3-point Likert scale (Not at all, Sometimes, A lot). Children ages 8 to 12 and adolescents ages 13 to 17 years were asked to rate how much each item has been a problem over the past 7 days on a 5-point Likert scale (Never, Almost Never, Sometimes, Often, Almost Always). The multidimensional scales include physical, emotional, social, and school functioning, and three summary scores include a total scale score, physical health summary score, and psychosocial health summary score. Items are reverse-scored and linearly transformed to a 0 to 100 scale; thus, higher scores indicate better QOL. Previous studies reported internal consistency reliabilities of 0.88 for child reports [10] and excellent validity [11,12].
Follow-up Child Open-Ended Questions
Children in the intervention group completed individual interviews at T2 with open-ended questions about what they liked and did not like about the activity. Graduate student RAs conducted the interviews in person after administering the T2 PedsQL. Children’s responses were audio-recorded and transcribed verbatim.
Follow-up Parent Survey
An advanced practice research nurse (who had not previously interacted with participants) called parents of children who completed the intervention, inviting them to answer electronic survey questions regarding effects of the intervention and suggestions for future research. The nine survey questions and multiple choice answers were developed by the research team and are listed verbatim in Table III. Some multiple choice answers included “other” where participants could offer open-ended comments. Two PhD prepared research nurses and a physician scientist, all with experience in our preliminary research on legacy-making, reviewed the survey for face validity. Parents could complete the electronic survey independently or have the research nurse read the questions to them and record their responses over the phone.
Table III.
Counts and frequencies of parent responses to follow-up survey
| Why did you allow your child to participate in our project? | |
| I thought the study results could benefit other families in the future | 16 (66.7) |
| I thought it would help my child | 8 (33.3) |
| I thought it would help our family | 5 (20.8) |
| I wanted a video of my child | 2 (8.3) |
|
| |
| Why do you think your child wanted to participate? | |
| My child thought it would be a fun activity | 15 (62.5) |
| My child wanted an opportunity to help others | 11 (45.8) |
| My child wanted to create a memory/legacy | 8 (33.3) |
| My child wanted to make something to give to someone special | 2 (8.3) |
| Other | 1 (4.2) |
|
| |
| Since your child completed participation in our project, have you or has your child done anything with the video? | |
| We have watched the video again | 18 (75.0) |
| Other | 5 (20.8) |
| We gave the video to someone special | 3 (12.5) |
| We have never watched the video again | 2 (8.3) |
|
| |
| How was/is the video helpful to you? | |
| Provides emotional comfort to me | 11 (45.8) |
| The process of making the video facilitated communication between me and my child | 9 (37.5) |
| Is a coping strategy for me | 4 (16.7) |
| The video does not help me | 0 (0.0) |
|
| |
| How was/is the video helpful to your child? | |
| Helped my child express his/her feelings | 19 (79.2) |
| Helped my child cope | 6 (25.0) |
| Other | 6 (25.0) |
| Helped my child feel better emotionally | 5 (20.8) |
| Helped my child feel better socially | 3 (12.5) |
| Helped my child feel better physically | 2 (8.3) |
| Helped my child feel better spiritually | 2 (8.3) |
| The video was not helpful to my child | 0 (0.0) |
|
| |
| How was/is the video unhelpful to you or your child? | |
| Made our family feel sad | 3 (15.0) |
| Other: “Never received it” “It makes me question why this happened to her.” “I have mixed emotions. When she was alive, I loved the video. Now, I get sad because I miss her.” |
3 (13.6) |
| Made me feel sad | 1 (5.0) |
| Made my child feel sad | 0 (0.0) |
|
| |
| How do you think the process would be more helpful to other families in the future? | |
| Structure the videotaping so that parents and their children create the video together, perhaps with parents asking the children questions rather than a nurse researcher. | 8 (36.4) |
| Structure the videotaping so that children create the video with a sibling. | 6 (27.3) |
| Structure the videotaping so that children create the video alone, without a family member but with the assistance of a nurse. | 4 (18.2) |
|
| |
| In your opinion, when is the best time to offer this activity? | |
| During treatment | 12 (50) |
| After treatment | 8 (33.3) |
| Other | 8 (33.3) |
| Soon after diagnosis | 3 (12.5) |
|
| |
| Do you still have the video? | |
| Yes | 16 (66.7) |
| No | 8 (33.3) |
Analysis
Frequency distributions and Chi-Square Tests of Independence were used to summarize and compare the nominal demographic characteristics of the study groups. Normally distributed continuous data (age, education) were summarized using mean and standard deviation; median and 25th–75th interquartile range summarized skewed data (time since diagnosis). These data were compared using independent t-tests and the Mann-Whitney test. Graphical and descriptive statistical summaries (mean, SD) of the PedsQL at both times of assessment, as well as changes in values from T1 to T2, were evaluated. To control for differences in baseline values of the PedsQL scores, differences between the groups in change in QOL were evaluated using analysis of covariance that included the respective PedsQL baseline score as the covariate. Cohen’s d effect sizes were also generated. While the focus was on generating reliable estimates of outcome values and differences in those values, all tests of statistical significance used two-sided type I error rates (alpha values) of no more than 0.05. These summaries and analyses were conducted using SPSS (22.0). Two researchers independently analyzed data from open-ended responses via qualitative content analysis [13,14]. The two researchers coded data individually and then discussed emerging themes until reaching consensus. Counts and frequencies were calculated for multiple choice survey responses.
RESULTS
Of 36 eligible child-parent dyads, 28 (78%) participated in the study. One child participant did not complete the study due to death. Those who did not participate typically included a child or parent verbalized they simply were “not interested.” The majority of participating parent caregivers were female (n = 23, 82%), married (n = 17, 61%), biological parents (n = 27, 96%), Caucasian (n = 20, 74%), and non-Hispanic (n = 25, 89%). They averaged 36 years of age (SD = 4.30). The majority reported a family income of under $25,000 per year (n = 10, 37%). Most parents identified themselves as very spiritual (n = 16, 59%) and attended religious services once a month or less (n = 12, 43%). The ill children averaged 11 years of age (range 7–17). The majority were female (n = 15, 54%), Caucasian (n = 21, 75%), and non-Hispanic (n = 25, 89%). The time since children had been diagnosed with cancer averaged 18 months, ranging from 3 to 58 months. Twenty-two percent of children (n = 6) had experienced a recurrence or progression of his or her disease post-treatment. See Table I for complete information on participant and disease characteristics.
Table I.
Group demographic and treatment characteristics
| Parents | Total Sample (N = 28) | Intervention (n = 15) | Control (n = 13) | Intervention vs. Control | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | M | SD | M | SD | P-Value | |
|
| |||||||
| Age | 36.21 | 4.298 | 34.93 | 4.480 | 37.69 | 3.706 | 0.090 |
|
| |||||||
| Highest grade of school completed | 16.64 | 3.664 | 17.13 | 3.091 | 16.08 | 4.291 | 0.457 |
|
| |||||||
| Gender n (%) | 0.502 | ||||||
| Male | 5 | 17.9% | 2 | 13.3% | 3 | 23.1% | |
| Female | 23 | 82.1 | 13 | 86.7 | 10 | 76.9 | |
|
| |||||||
| Marital status | 0.224 | ||||||
| Single | 4 | 14.3 | 2 | 13.3 | 2 | 15.4 | |
| Married | 17 | 60.7 | 9 | 60.0 | 8 | 61.5 | |
| Divorced | 2 | 7.1 | 2 | 13.3 | 0 | 0.0 | |
| Separated | 2 | 7.1 | 0 | 0.0 | 2 | 15.4 | |
| Remarried | 2 | 7.1 | 2 | 13.3 | 0 | 0.0 | |
| Living with someone | 1 | 3.6 | 0 | 0.0 | 1 | 7.7 | |
|
| |||||||
| Annual family income | 0.290 | ||||||
| < $25,000 | 10 | 37.0 | 5 | 35.7 | 5 | 38.5 | |
| $25,001–$50,000 | 5 | 18.5 | 1 | 7.1 | 4 | 30.8 | |
| $50,001–$75,000 | 5 | 18.5 | 3 | 21.4 | 2 | 15.4 | |
| $75,001–$100,000 | 3 | 11.1 | 3 | 21.4 | 0 | 0.0 | |
| $100,001 or more | 4 | 14.8 | 2 | 14.3 | 2 | 15.4 | |
|
| |||||||
| Relation to child | 0.343 | ||||||
| Biological parent | 27 | 96.4 | 14 | 93.3 | 13 | 100.0 | |
| Adoptive parent | 1 | 3.6 | 1 | 6.7 | 0 | 0.0 | |
|
| |||||||
| Race | 0.367 | ||||||
| Caucasian | 20 | 74.1 | 11 | 78.6 | 9 | 69.2 | |
| African American | 4 | 14.8 | 2 | 14.3 | 2 | 15.4 | |
| Asian | 1 | 3.7 | 1 | 7.1 | 0 | 0.0 | |
| Other | 2 | 7.4 | 0 | 0.0 | 2 | 15.4 | |
|
| |||||||
| Ethnicity | 0.049 | ||||||
| Hispanic or Latino | 3 | 10.7 | 0 | 0.0 | 3 | 23.1 | |
| Non-Hispanic | 25 | 89.3 | 15 | 100.0 | 10 | 76.9 | |
|
| |||||||
| Religion | 0.293 | ||||||
| Protestant | 3 | 10.7 | 3 | 20.0 | 0 | 0.0 | |
| Roman Catholic | 1 | 3.6 | 0 | 0.0 | 1 | 7.7 | |
| Jewish | 1 | 3.6 | 1 | 6.7 | 0 | 0.0 | |
| None | 6 | 21.4 | 3 | 20.0 | 3 | 23.1 | |
| Other | 17 | 60.7 | 8 | 53.3 | 9 | 69.2 | |
|
| |||||||
| Attendance at religious services | 0.833 | ||||||
| Several times/week | 5 | 17.9 | 2 | 13.3 | 3 | 23.1 | |
| Once/week | 5 | 17.9 | 3 | 20.0 | 2 | 15.4 | |
| 2–3 times/month | 5 | 17.9 | 3 | 20.0 | 2 | 15.4 | |
| Once a month/less | 12 | 42.9 | 6 | 40.0 | 6 | 46.2 | |
| Never | 1 | 3.6 | 1 | 6.7 | 0 | 0.0 | |
|
| |||||||
| Spirituality | 0.819 | ||||||
| Not spiritual at all | 1 | 3.7 | 1 | 6.7 | 0 | 0.0 | |
| Not very spiritual | 2 | 7.4 | 1 | 6.7 | 1 | 8.3 | |
| Fairly spiritual | 8 | 29.6 | 4 | 26.7 | 4 | 33.3 | |
| Very spiritual | 16 | 59.3 | 9 | 60.0 | 7 | 58.3 | |
|
| |||||||
| Child | |||||||
|
| |||||||
| Race | 0.733 | ||||||
| Caucasian | 21 | 75 | 12 | 80.0 | 9 | 69.2 | |
| African American | 4 | 14.3 | 2 | 13.3 | 2 | 15.4 | |
| Other | 3 | 10.7 | 1 | 6.7 | 2 | 15.4 | |
|
| |||||||
| Ethnicity | 0.457 | ||||||
| Hispanic or Latino | 3 | 10.7 | 1 | 6.7 | 2 | 15.4 | |
| Non-Hispanic | 25 | 89.3 | 14 | 93.3 | 11 | 84.6 | |
|
| |||||||
| Time since diagnosis (months) | 18.15 | 21.10 | 15.43 | 0.084 | |||
|
| |||||||
| Recurrence/progression of disease post-treatment | 0.410 | ||||||
| No | 21 | 77.8 | 10 | 71.4 | 11 | 84.6 | |
| Yes | 6 | 22.2 | 4 | 28.6 | 2 | 15.4 | |
| Time since recurrence (months) | 3.58 | 5.28 | 3.58 | 1.000 | |||
| Tumor resection surgery | |||||||
| Yes | 1 | 16.7 | 4 | 100.0 | 1 | 50.0 | |
| No | 5 | 83.3 | 0 | 0.0 | 1 | 50.0 | |
| Chemotherapy | 6 | 100.0 | 4 | 100.0 | 2 | 100.0 | |
| Radiotherapy | |||||||
| Yes | 4 | 66.7 | 2 | 50.0 | 2 | 100.0 | |
| No | 2 | 33.3 | 2 | 50.0 | 0 | 0.0 | |
| Bone marrow transplant | |||||||
| Yes | 2 | 33.3 | 2 | 50.0 | 0 | 0.0 | |
| No | 4 | 66.7 | 2 | 50.0 | 2 | 100.0 | |
| Phase 1 studies | |||||||
| Yes | 1 | 16.7 | 1 | 25.0 | 0 | 0.0 | |
| No | 5 | 83.3 | 3 | 75.0 | 2 | 100.0 | |
| Physician noted disease is terminal | |||||||
| Yes | 1 | 16.7 | 1 | 25.0 | 0 | 0.0 | |
| No | 5 | 83.3 | 3 | 75.0 | 2 | 100.0 | |
| Hospice referral | |||||||
| Yes | 1 | 16.7 | 1 | 25.0 | 0 | 0.0 | |
| No | 5 | 83.3 | 3 | 75.0 | 2 | 100.0 | |
Twenty-seven of 28 (96%) child participants completed the entire study. The one attrition was due to death. Descriptive summaries of the PedsQL scores at baseline (T1) and end-of-study (T2) are shown in Table II. The two groups were very similar at baseline for the emotional and school functioning scales yet quite different on physical and social functioning. While the difference in social functioning was not statistically significant, the higher physical function level of the intervention group compared to controls at baseline was statistically significant (M = 85, SD = 15 vs. M = 66, SD = 22; p = 0.024) (see Table II). Therefore, while the Cohen’s d effect size for change (worsening) in physical functioning was considerably larger in the intervention group (−0.63) compared to controls (−0.03), the difference between the groups in physical functioning change was not statistically significant. Differences in effect sizes for emotional, social, or school functioning were not as large as for physical and not statistically significant, yet the intervention group showed slightly better emotional and school functioning compared to controls.
Table II.
Summaries of PedsQL scores at baseline and end-of-study
| QOL (PedsQL) | T1 | T2 | p-value | Effect Size* | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Physical functioning | 0.135 | |||||
| Control (n=13) | 66.41 | 22.36 | 65.69 | 17.82 | −0.03 | |
| Intervention (n=14) | 84.76 | 14.97 | 70.98 | 21.87 | −0.63 | |
| Emotional | 0.685 | |||||
| Control (n=13) | 77.88 | 11.17 | 76.92 | 20.16 | −0.05 | |
| Intervention (n=14) | 77.86 | 22.68 | 80.36 | 15.75 | 0.11 | |
| Social | 0.717 | |||||
| Control (n=13) | 82.50 | 22.45 | 74.23 | 17.30 | −0.37 | |
| Intervention (n=14) | 77.86 | 22.16 | 76.43 | 21.96 | −0.06 | |
| School | 0.710 | |||||
| Control (n=12) | 61.98 | 21.80 | 65.31 | 17.74 | 0.15 | |
| Intervention (n=14) | 67.86 | 27.72 | 74.29 | 18.69 | 0.23 | |
| Total | 0.825 | |||||
| Control (n=12) | 70.08 | 13.71 | 70.01 | 11.49 | −0.01 | |
| Intervention (n=14) | 77.08 | 18.11 | 75.51 | 13.18 | −0.09 | |
Cohen’s d estimate
Children in the intervention group (n = 14) completed open-ended questions at T2 regarding what they liked and disliked about the intervention. One overall theme emerged from the data, which was that the children perceived the intervention as fun and enjoyable. All children (n = 14, 100%) reported that they liked the digital storytelling activity, especially the music and photographs. One child said, “They came to my house and let me make a video about me. I got to show them my pets, talk all about me and stuff I like. Now it’s on Facebook and I can watch it with my mom and friends whenever I want to. It was so much fun!” The majority of children reported that there was nothing they disliked about the activity. Only a few children commented on things they did not like, such as one child who reported he/she did not like reading. Another child said he/she liked two of the photographs used but not the others, commenting that other people had selected the photographs.
Primary parent caregivers (N = 22) of the children who completed the intervention, including both the intervention and control group (controls completed the intervention post-T2), answered survey questions regarding effects of the intervention and suggestions for future research. The majority of parents allowed their children to participate in hopes that study results would benefit other families (n = 16; 67%), while other parents felt participation would help their child (n = 8, 33%) and/or their family (n = 5, 21%). Parents perceived that children chose to participate mainly because their child thought it would be a fun activity (n = 15, 63%), wanted an opportunity to help others (n = 11, 46%), and wanted to create a memory/legacy (n = 8, 33%). Many parents reported that their child’s digital story helped their children express their feelings (n = 19; 79%) and provided emotional comfort to parents (n = 11, 46%). Some parents perceived that the intervention facilitated communication between parents and children (n = 9; 38%), was a parent coping strategy (n = 4; 17%), and helped children cope (n = 6, 27%), feel better emotionally (n = 5, 23%), socially (n = 3, 13%), physically (n = 2, 9%), and spiritually (n = 2, 9%). One bereaved parent reported that her child “…wanted to do [the intervention]. This was the only [study] she was determined to participate in…” Seventy-five percent (n = 18) of parents reported that their family has watched their child’s digital story again. Forty-five percent (n = 10) of parents shared the digital story with others. Digital stories have averaged 258 views per YouTube video to date (can only be viewed by individuals who have the web link from the family), ranging from 4 to 4832 views. One bereaved parent showed the video at the child’s funeral.
DISCUSSION
This research represents the only study to date that directly proposes legacy-making as a possible strategy to improve QOL for children with cancer. Previous pediatric interventions have indirectly incorporated legacy-making components, primarily via music therapy interventions [15–17]. While these previous interventions have been shown to decrease distress and improve coping for pediatric palliative care patients, they are limited to music-related intervention delivery formats and indirect legacy-making content. Our intervention, developed via child and parent self-reports [6], complements but expands previous work by directly focusing on legacy-making and using digital storytelling that incorporates audio, video, music, and photographs, allowing for greater individualization to each child and family. Results advanced our understanding of how legacy-making may influence QOL outcomes in children living with cancer and provide a strong foundation for full-scale testing of our newly developed intervention.
Our 78% (28 of 36) participation rate, 96% (27 of 28) retention of families at T2, and 79% (22 of 28) retention of families for the parent follow-up survey (T3) support the willingness of these children and parents to participate, despite advanced illness and even potentially being near the end of life, and provide strong evidence for feasibility. Participant incentives (parent-child dyads received $20 at T1 and T2; parents completing the follow-up survey received $50 Visa giftcards), reminder calls, and scheduling appointments at convenient times and locations for families likely reduced dropout. Strong completion rates and positive child and parent feedback suggest our intervention is developmentally appropriate for children 7 to 17 years of age, with any type of cancer and stage of illness. Children with cancer (including relapsed or refractory cancer) near the end of life completed our intervention with ease. One 7-year-old participant completed the intervention soon after beginning hospice care. A relapse patient completed the intervention 61 days before death, and several others completed the intervention 3 to 4 months before death. Advanced cancer patients may be most likely to benefit from a legacy-making intervention as noted by adult studies [7]. More work is needed to determine pediatric patient subgroups most likely to benefit from legacy-making.
Results support preliminary intervention efficacy and value. Quantitative data suggested that the emotional component of QOL may be the area showing the most promise to benefit children. The follow-up parent interviews provided invaluable insight to additional benefits not only to ill children, but also for parents. Parents strongly endorsed that the intervention could benefit other families, was fun for children, and helped children express his or her feelings. On the contrary, very few parents perceived the intervention as a coping strategy or that it improved children’s social, physical, or spiritual well-being. This is interesting, particularly in light of the fact that 79% of parents perceived that it allowed children to express their feelings. Further, children’s hospital staff have reported that legacy-making activities helped ill children and their family members cope and communicate [9]. Child and parent coping and adjustment outcomes, including emotional functioning and communication, may be ideal outcomes to examine in future work. It will also be important to gather self-reports from both children and their parents, as parent perceptions may not accurately reflect child perceptions.
Only two (8.3%) parents perceived that their children participated to make or give something to someone special, and only three (12.5%) families reported giving the video to someone special. This differs from the generativity dimension of legacy-making seen in adult participants [7]. Adults who have participated in legacy-making interventions may have done so partly because of the dignity therapy implication of generativity that involves bequeathing their legacy-making document to a friend or family members. While most components of the dignity therapy model of palliative care (continuity of self, role preservation, maintenance of pride, hopefulness, and aftermath concerns) previously tested in adults seem to naturally align with our pediatric legacy-making intervention, generativity may be different in children compared to adults. This could be due in part to children’s developmental understanding of death. Children generally begin to understand that death is permanent and universal during Piaget’s concrete operational stage, which usually begins at 7 years of age. Previous work has found most bereaved family members perceived that children (averaging 12 years of age) living with advanced cancer were aware of his or her impending death [5]. While some family members reported that very young children (e.g., 3 years of age) explicitly wanted to be remembered and create a legacy, most portrayed awareness of impending death through implied words or behaviors. Perhaps the concept of generativity is present in dying children yet displayed or articulated in different ways compared to adults. More work is needed to better understand generativity in children living with life-threatening illnesses.
Of the 22 parents who completed the follow-up survey, five (22.7%) had experienced the death of their child at the point of the survey. All five (100%) bereaved parents agreed to complete the parent follow-up study. Previous studies have contacted bereaved families as soon as three months after the death of a child [18]. In our study, we contacted bereaved parents as early as six weeks post-death. This was due in part because we had positive established relationships with them and maintained communication. In longitudinal studies beginning during a child’s illness, it may be appropriate to continue data collection with bereaved parents as soon as six weeks post-death when positive relationships are established and maintained. However, researchers must be sensitive to the individual nature of grief and recognize that it may not be best for every bereaved parent to continue on study.
Generalizability of results are limited to school-aged and adolescent children who have cancer and their parents. Participants included children with cancer diagnoses; thus, the intervention may not be feasible or applicable to children with other life-threatening conditions. The varied cancer diagnoses among our sample may have created different physical and mental issues, and our sample spanned across school-age and adolescent development stages. The majority of participating parents were mothers, so parent perspectives may not generalize to fathers. Ethnicity differed significantly between the intervention and control arms, possibly influencing study results. Attentional effects of the intervention could also have influenced study results; future studies may address this by controlling for face-to-face time with a research nurse. However, this study demonstrated the feasibility of this legacy-making intervention within this clinical setting.
Despite these limitations, our legacy-making intervention for children with cancer is a feasible and promising strategy to improve coping and adjustment for ill children and their families. We are now well-positioned to examine efficacy of our intervention for children with relapsed or refractory cancer and their parents. If effective, we envision comparative effectiveness testing between larger sites with widespread dissemination to children’s hospitals nationwide. Future work should determine the best point in the illness trajectory to offer legacy-making interventions, as well as populations for whom the intervention would be most beneficial. Research is needed to better understand how legacy-making may impact younger patients (e.g., toddlers, preschoolers) and other family members (e.g., siblings, grandparents). We hope to further build this body of literature to advance the field of pediatric oncology and palliative care and ultimately decrease suffering and enhance life for children living and suffering from cancer.
Acknowledgments
The authors would like to thank the families who generously participated in this work. This research was supported by a grant from the Robert Wood Johnson Foundation Nurse Faculty Scholar program awarded to Dr. Terrah Foster Akard 2010–2013 (Grant ID# 68045) and NCATS/NIH grant support (UL1 TR000445).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. [Accessed August 14, 2014];Cancer Facts and Figures. 2014 http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf.
- 2.National Cancer Institute. [Accessed January 28, 2009];SEER Cancer Statistics Review 1975–2005. Table XXVIII-12. http://seer.cancer.gov/csr/1975_2005/results_merged/sect_28_childhood_cancer.pdf.
- 3.Kane JR, Primomo M. Alleviating the suffering of seriously ill children. Am J Hosp Palliat Care. 2001;18(3):161–169. doi: 10.1177/104990910101800307. [DOI] [PubMed] [Google Scholar]
- 4.Hinds PS, Drew D, Oakes LL, Fouladi M, Spunt SL, Church C, Furman WL. End-of-life care preferences of pediatric patients with cancer. J Clin Oncol. 2005;23(36):9146–9154. doi: 10.1200/JCO.2005.10.538. [DOI] [PubMed] [Google Scholar]
- 5.Foster TL, Gilmer MJ, Davies B, Barrera M, Fairclough D, Vannatta K, Gerhardt CA. Bereaved parents’ and siblings’ reports of legacies created by children with cancer. J Pediatr Oncol Nurs. 2009;26(6):369–376. doi: 10.1177/1043454209340322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akard TF, Gilmer MJ, Friedman DL, Given B, Hendricks-Ferguson VL, Hinds PS. From qualitative work to intervention development in pediatric oncology palliative care research. J Pediatr Oncol Nurs. 2013;30(3):153–160. doi: 10.1177/1043454213487434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chochinov HM, Hack T, Hassard T, Kristjanson LJ, McClement S, Harlos M. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520–5525. doi: 10.1200/JCO.2005.08.391. [DOI] [PubMed] [Google Scholar]
- 8.Coyle N. The hard work of living in the face of death. J Pain Symptom Manage. 2006;32(3):266–274. doi: 10.1016/j.jpainsymman.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Foster TL, Dietrich MS, Friedman DL, Gordon JE, Gilmer MJ. National survey of children’s hospitals on legacy-making activities. J Palliat Med. 2012;15(5):573–578. doi: 10.1089/jpm.2011.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hinds PS, Billups CA, Cao X, Gattuso JS, Burghen E, West N, Rubnitz JE, Daw NC. Health-related quality of life in adolescents at the time of diagnosis with osteosarcoma or acute myeloid leukemia. Eur J Oncol Nurs. 2009;13(3):156–163. doi: 10.1016/j.ejon.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung L, Yanofsky R, Klaassen RJ, Dix D, Pritchard S, Winick N, Alexander S, Klassen A. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer. 2011;128(5):1213–1220. doi: 10.1002/ijc.25433. [DOI] [PubMed] [Google Scholar]
- 13.Hickey G, Kipping C. A multi-stage approach to the coding of data from open-ended questions. Nurse Res. 1996;4(1):81–91. doi: 10.7748/nr.4.1.81.s9. [DOI] [PubMed] [Google Scholar]
- 14.LoBiondo-Wood G, Haber J. Nursing research: Methods and critical appraisal for evidence-based practice. 6. St. Louis, MO: Mosby Elsevier; 2006. [Google Scholar]
- 15.Burns DS, Robb SL, Haase JE. Exploring the feasibility of a therapeutic music video intervention in adolescents and young adults during stem-cell transplantation. Cancer Nursing. 2009;32(5):E8–E16. doi: 10.1097/NCC.0b013e3181a4802c. [DOI] [PubMed] [Google Scholar]
- 16.Robb SL, Burns DS, Stegenga KA, Haut PR, Monahan PO, Meza J, Stump TE, Cherven BO, Docherty SL, Hendricks-Ferguson VL, Kintner EK, Haight AE, Wall DA, Haase JE. Randomized clinical trial of therapeutic music video intervention for resilience outcomes in adolescents/young adults undergoing hematopoietic stem cell transplant: a report from the Children’s Oncology Group. Cancer. 2014;120(6):909–917. doi: 10.1002/cncr.28355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robb SL, Ebberts AG. Songwriting and digital video production interventions for pediatric patients undergoing bone marrow transplantation, part I: an analysis of depression and anxiety levels according to phase of treatment. J Pediatr Oncol Nurs. 2003;20(1):2–15. doi: 10.1053/jpon.2003.3. [DOI] [PubMed] [Google Scholar]
- 18.Gerhardt CA, Fairclough DL, Grossenbacher JC, Barrera M, Gilmer MJ, Foster TL, Compas BE, Davies B, Hogan NS, Vannatta K. Peer relationships of bereaved siblings and comparison classmates after a child’s death from cancer. J Pediatr Psychol. 2012;37(2):209–219. doi: 10.1093/jpepsy/jsr082. [DOI] [PMC free article] [PubMed] [Google Scholar]


