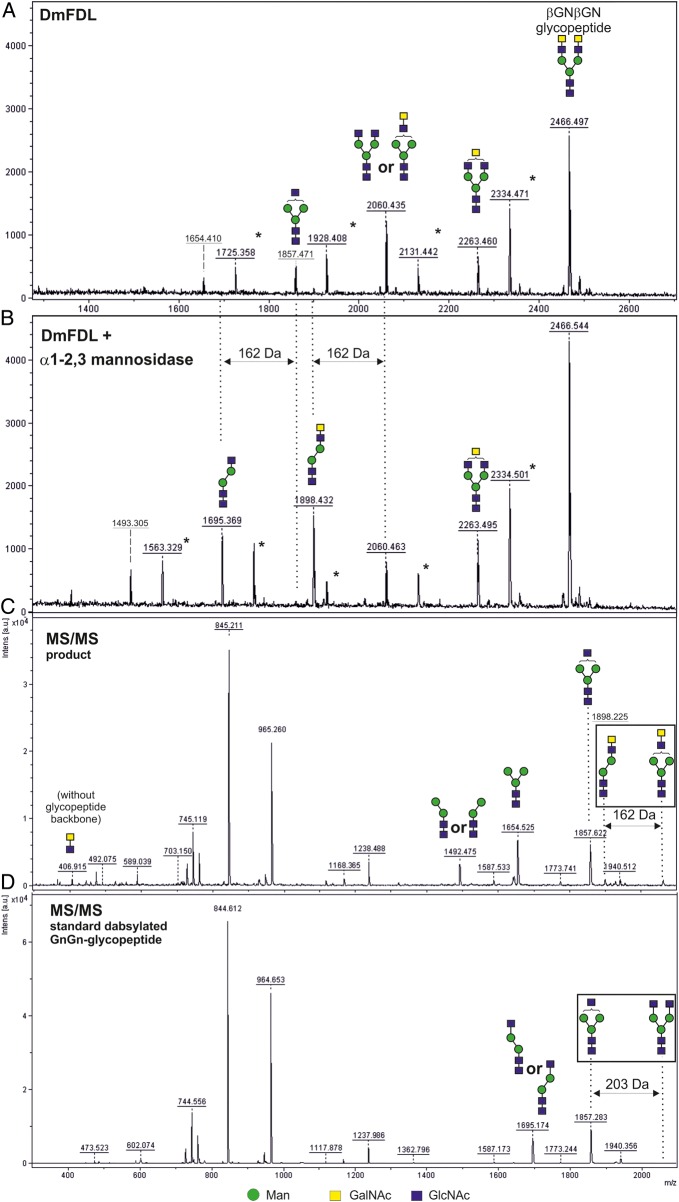
Fig. 6.
The recombinant DmFDL can process N-glycan substrates carrying terminal LacdiNAc. The dabsylated-βGNβGN glycopeptide (containing two terminal β-linked GalNAc residues) was partially processed in the presence of excess of the DmFDL enzyme produced in P. pastoris over an extended period of 3 days (A). The products were incubated in the presence of α1,3-mannosidase, which facilitated the removal of a terminal, α1,3-linked mannose residue indicating that a part of the DmFDL products are structures lacking one GlcNAc and one GalNAc residues, instead of two GalNAc residues (B). This result is corroborated by the presence of the ion with m/z 1898 in the MS/MS spectra of the relevant product (the structure with m/z 2060) (C). In contrast, the MS/MS spectrum of the dabsylated GnGn-glycopeptide carrying one GlcNAc residue on each arm of the biantennary N-glycan (the structure with the same m/z of 2060) contains neither the ion with m/z 407 (corresponding to two linked HexNAc residues) nor the ion with m/z 1898 (D). All structures were detected in the [M+H+] form. The glycans are depicted following the glycan nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org). Asterisk indicates the peaks derived from the laser-induced removal of the dabsyl group from the dabsylated glycopeptides.