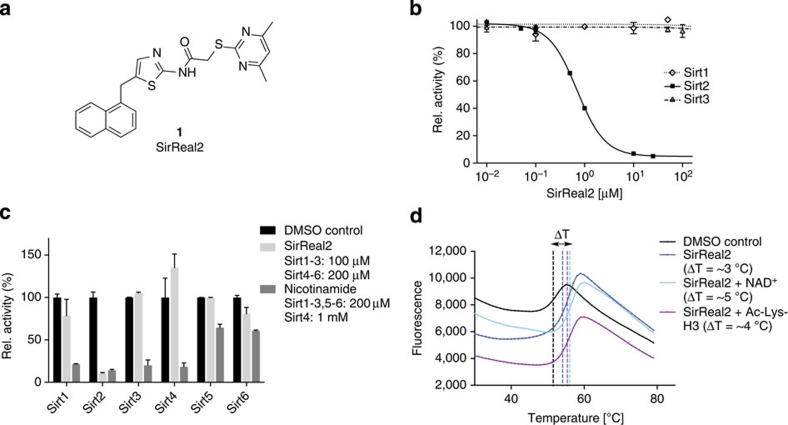
Figure 1. SirReal2 selectively inhibits Sirt2 in a dose-dependent manner.
(a) Chemical structure of SirReal2 (1). (b) Representative dose–response curve for Sirt1–3 and SirReal2 using the substrates ZMAL (Z-Lys(Acetyl)-AMC, Sirt1-2) resp. Fluor-de-Lys (Sirt3). Compared with the peptide-HPLC assay, SirReal2 was slightly less potent using ZMAL with an IC50 value of 0.4 μM. Data are presented as mean±s.d. (n=3). (c) In vitro inhibition data for SirReal2 (Sirt1–3: 100 μM; Sirt4–6: 200 μM) in an assay using non-labelled acyl-lysine oligopeptide as a substrate (Sirt1–4, acetyl-lysine substrate; Sirt5, succinyl-lysine substrate; Sirt6, myristoyl-lysine substrate). A solution containing DMSO was used as a negative control, a solution with nicotinamide (NCA, 200 μM or 1 mM) was used as a positive control. Only the activity of Sirt2 is substantially reduced in the presence of SirReal2. Data are presented as mean±s.d. (n=2) (d) Representative thermal stability plots for Sirt2 in the presence of SirReal2 (25 μM) and either the cofactor NAD+ (5 mM) or an acetyl-lysine H3 peptide (5 mM). The presence of NAD+ or of an acetyl-lysine peptide substrate enhances the stability of the Sirt2–SirReal2 complex (n=3). Representative thermal stability plots of Sirt2 in the absence of SirReal2 and in the presence of NAD+ or an acetyl-lysine oligopeptide are shown in Supplementary Fig. 1d. Rel., relative.